ABSTRACT
From 6% to 8% of patients who present with myocardial infarction have no evidence of obstructive coronary artery disease on angiography. This subgroup tends to be younger, and more of them are women. This review highlights a proposed algorithm to identify the underlying cause of myocardial infarction with nonobstructive coronary arteries (MINOCA). We emphasize the need for a collaborative approach in diagnosing and managing MINOCA to improve patient outcomes, advocating for a standardized diagnostic pathway that incorporates cardiac magnetic resonance imaging and comprehensive clinical evaluation to tailor treatments effectively.
The diagnosis of myocardial infarction requires a rise or fall in troponin plus other evidence of acute ischemia such as symptoms, electrocardiographic changes, imaging evidence, or a thrombus detected on coronary angiography.
MINOCA is a subtype of myocardial infarction in which there is no significant epicardial stenosis.
MINOCA has multiple potential causes, and additional clinical evaluation and testing are required to determine which one the patient has. However, data are limited regarding subtype-specific treatment and prognosis.
Intracoronary imaging and cardiac magnetic resonance imaging are key tests in the diagnosis of MINOCA.
A 53-year-old man with a history of human immunodeficiency virus infection, on antiretroviral therapy, was brought to the emergency department by ambulance with acute onset of substernal chest tightness. His initial electrocardiogram showed normal sinus rhythm with borderline inferior-wall ST-segment elevation, which did not, however, meet the criteria for ST-segment elevation myocardial infarction (STEMI) (Figure 1). The ST-segment elevation was unchanged on repeat tracing.
The patient’s initial electrocardiogram showing borderline ST-segment elevation, which did not, however, meet the criteria for ST-segment elevation myocardial infarction (ie, ST-segment elevation ≥ 1 mm [0.1 mV] above the baseline in at least 2 contiguous leads [except V2 and V3]).
The patient’s chest discomfort resolved after he received aspirin, sublingual nitroglycerin, and 4,000 units of intravenous unfractionated heparin. His initial troponin I level was 0.49 ng/mL (upper reference limit 0.030 ng/mL), and it increased to 8.4 ng/mL at 2 hours and 14.7 ng/mL at 6 hours after the initial measurement, which prompted an urgent referral to the cardiac catheterization laboratory.
No obstructive epicardial coronary artery disease (≥ 50% stenosis) or evidence of plaque rupture was noted on initial angiography (Figure 2). There was, however, a moderate narrowing in the first septal perforator branch of the left anterior descending artery. Transthoracic echocardiography showed a regional wall-motion abnormality in the mid-anterior and mid-inferior septum.
Coronary angiography. On the left, a right anterior oblique caudal view of the left coronary artery, and on the right, a left anterior oblique caudal view of the right coronary artery (RCA), showing no obstructive lesions in the main branches of either.
Cx = circumflex; LAD = left anterior descending; LM = left main; PL = posterolateral; PDA = posterior descending artery
In view of his symptoms and initial evaluation suggestive of myocardial infarction but without significant stenosis or culprit lesion on angiography, we gave him a provisional diagnosis of myocardial infarction with nonobstructive coronary arteries (MINOCA).
MYOCARDIAL INFARCTIONS DEFINED AND CLASSIFIED
According to the Fourth Universal Definition of Myocardial Infarction,1 published in 2018, the diagnosis of myocardial infarction requires myocardial injury and additional evidence of acute myocardial ischemia. Myocardial injury is an umbrella term for all clinical scenarios, regardless of etiology, in which the cardiac troponin level is higher than the 99th percentile upper reference limit. Cardiac troponin I is strongly preferred over troponin T because the former is absent in noncardiac myocytes, so it has higher sensitivity and specificity.
Myocardial infarction is considered acute when there is a rise or fall or both in troponin I, whereas chronic myocardial injury is characterized by elevated cardiac troponin values with variation in troponin values of 20% or less.1 Thus, myocardial infarction is a subtype of myocardial injury, with rise or fall of troponin I, due to an ischemic etiology, and therefore must be coupled with ischemic symptoms or objective findings on electrocardiography, noninvasive imaging, or cardiac catheterization.
Type 1 vs type 2. The Universal Definition1 recognizes 5 classes of myocardial infarction. Most type 1 cases result from occlusive or flow-limiting coronary thrombosis after atheromatous plaque rupture or erosion.2 Type 2 myocardial infarction, in contrast, occurs when myocardial oxygen demand outweighs supply (without plaque rupture), resulting in ischemia. Profound sepsis, significant anemia, or persistent tachyarrhythmia are examples of such supply-demand mismatch.
Type 3 involves sudden cardiac death occurring before cardiac troponin can be measured, and types 4 and 5 are iatrogenic. We won’t discuss these further here.
STEMI vs NSTEMI. Depending on the accompanying electrocardiographic changes, myocardial infarctions are also classified as either non-ST segment elevation (NSTEMI) or STEMI. Frequently, STEMI is associated with an acutely and totally occluded artery, whereas NSTEMI is associated with a severe luminal narrowing but with some residual flow. STEMI and NSTEMI are the most common types of type 1 myocardial infarction. While type 2 myocardial infarction is not typically associated with the traditional mechanisms of coronary obstruction seen in type 1 myocardial infarction (where a plaque rupture leads to thrombosis), it can still result in ST-segment elevation on an electrocardiogram.
Obstructive vs MINOCA. Myocardial infarctions can occur without significant epicardial obstructive coronary disease (not clearly type 1 or type 2), a situation termed MINOCA.3–6 Approximately 6% to 8% of patients presenting with acute myocardial infarction can be classified as having MINOCA.7
Just as the type 1–vs–type 2 classification scheme has caused confusion among clinicians, as it cannot be directly transposed onto our traditional electrocardiogram-based classification scheme (STEMI vs NSTEMI), MINOCA adds another layer of complexity. In current clinical practice, if coronary angiography doesn’t show anything wrong, the evaluation may end there and a patient may be given a variety of diagnoses: NSTEMI, type 2 myocardial infarction, “troponin leak,” or no diagnosis, as it is incorrectly presumed that a myocardial infarction cannot occur if the coronary arteries are “clean.”
MINOCA AS A CLINICAL CONDITION
To be diagnosed with MINOCA, patients must satisfy the diagnostic criteria for myocardial infarction,1 and must have no epicardial obstructive disease on coronary angiography. Epicardial obstructive disease refers to lesions with angiographic diameter stenosis of at least 50%, though physiologic assessment is preferred in contemporary practice: the patient has epicardial obstructive disease if the fractional flow reserve is less than or equal to 0.80 and the instantaneous wave-free ratio is less than or equal to 0.89.8
Patients are younger and more often female, Black, or Hispanic
Patients with MINOCA are on average younger and more of them are women compared with patients with acute myocardial infarction due to obstructive coronary artery disease.5,6 In the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) registry,9 women were nearly 5 times more likely than men (odds ratio 4.8) to present with MINOCA. Women account for nearly 50% of patients with MINOCA but only 25% of those with myocardial infarction due to obstructive coronary artery disease. Compared with young women presenting with myocardial infarctions due to obstructive coronary artery disease, those with MINOCA were more likely to be premenopausal and less likely to have a history of gestational diabetes.9
Patients with MINOCA are also more likely to identify as Black, Hispanic, or Latino.6,9
A systematic review5 found a lower prevalence of dyslipidemia in patients with MINOCA, but other traditional risk factors appear similarly represented. Among young patients presenting with acute myocardial infarction in the VIRGO prospective registry, approximately 11% had MINOCA.9 Those with MINOCA were more likely to have a hypercoagulable state and more likely to have no traditional cardiac risk factors.
We used to assume that MINOCA had a lower mortality rate than myocardial infarction due to obstructive coronary artery disease, but we can’t be sure, as MINOCA was defined and diagnosed in different ways in different studies. Smilowitz et al6 reported a lower in-hospital mortality rate in patients with MINOCA than in those with myocardial infarction due to coronary artery disease (1.1% vs 2.9%). However, among young patients (18–55 years) in the VIRGO registry,9 the 2-month and 12-month mortality rates were similar for both groups.
The European Society of Cardiology issued a guideline paper on MINOCA in 2017,10 and the American Heart Association (AHA) followed in 2019.11 The AHA authors formalized and updated the definition of MINOCA in the hope that researchers will consistently use it, which could allow better characterization of the MINOCA population. They also proposed a diagnostic algorithm with the hope that it would lead to more effective management by excluding mimics of MINOCA and consistently identifying underlying etiologies (eg, plaque rupture, spontaneous coronary artery dissection, coronary vasospasm).
Here, we review the AHA guidelines for evaluating patients with MINOCA for the practicing clinician.
NUTS AND BOLTS OF THE AHA STATEMENT
The AHA scientific statement11 is pertinent to all hospitalized patients with myocardial infarction, although it presumes that everyone has access to imaging, including intravascular ultrasonography, optical coherence tomography, and cardiac magnetic resonance imaging. While the statement is geared toward practicing cardiologists, it is relevant for any hospital-based internist, internal medicine resident, or researcher.
The authors were primarily cardiologists; approximately half were interventional cardiologists, but there was also 1 hematologist and 1 PhD nurse scientist. The document reflects the consensus opinion of the authors and includes a comprehensive literature review, but the authors did not use a more formalized method for preparation such as the Delphi method.
The scientific statement was supported by the AHA, and its authors’ potential conflicts of interest are listed at the conclusion of the document. We note, without presumption of conflict, that 3 of the interventional cardiology providers have received consultancy dollars or grant funding from manufacturers of intravascular imaging equipment. We also observe that the recommended diagnostic algorithm strongly prefers newer technologies such as cardiac magnetic resonance imaging and optical coherence tomography, which may not be available at smaller healthcare facilities.
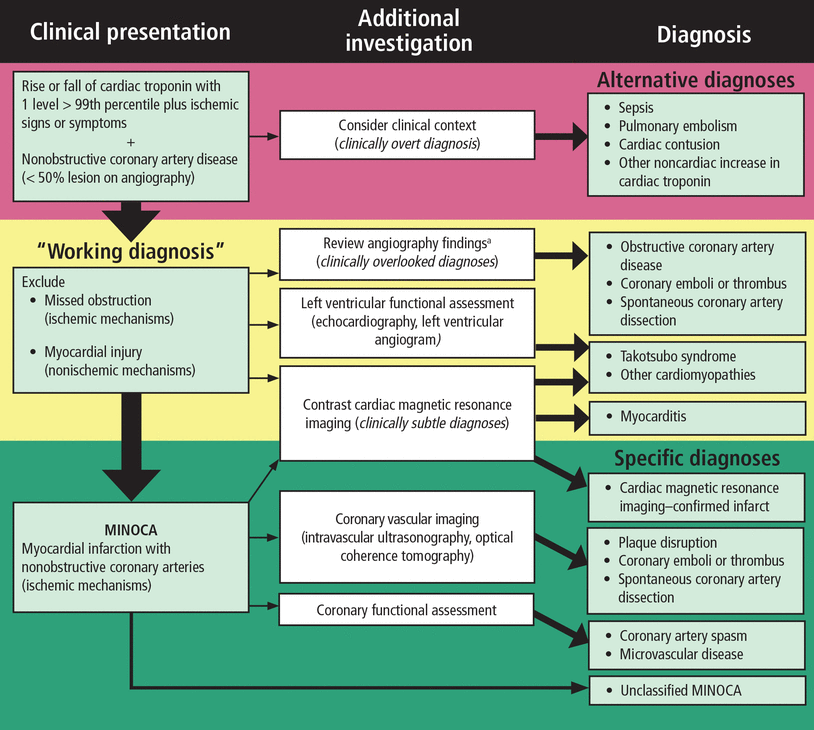
A TRAFFIC-LIGHT ALGORITHM FOR DIAGNOSING MINOCA
The AHA authors11 point out that MINOCA is a clinical syndrome that results from atherosclerotic and nonatherosclerotic mechanisms. They also emphasize that we lack high-quality, MINOCA-specific clinical studies on which to base therapy recommendations. That said, they propose a 3-step algorithm for diagnosing MINOCA, based on the analogy of a traffic light (Figure 3).11
American Heart Association “traffic light” algorithm for the diagnosis of myocardial infarction with nonobstructive coronary arteries (MINOCA). Red excludes nonischemic etiologies, yellow suggests slowing down to evaluate for alternate diagnoses that can mimic MINOCA, and green suggests a confirmed diagnosis of MINOCA.
aConsider fractional flow reserve.
Reprinted with permission from Tamis-Holland JE, Jneid H, Reynolds HR, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 2019; 139(18):e891–e908. doi:10.1161/CIR.0000000000000670. ©2019 American Heart Association, Inc.
Red light: First consider other diagnoses
Faced with a patient who has signs and symptoms suggesting ischemia and a rise in cardiac troponin but whose coronary arteries appear clean on angiography, the first step is to carefully review the clinical history to exclude “overt diagnoses” other than myocardial infarction. Examples include severe sepsis, massive pulmonary embolism, and severe anemia.
In these cases, no further cardiac diagnostic workup is recommended unless there is evidence of ischemia out of proportion to the degree of clinical illness. If there is no alternative diagnosis to explain a troponin elevation, it is reasonable to consider a working diagnosis of MINOCA.
Yellow light: Take a closer look at the angiogram
Once the clinical team reaches a working diagnosis of MINOCA, the next steps are to review the angiogram again and assess left ventricular function with echocardiography or assess the myocardium with cardiac magnetic resonance imaging with gadolinium contrast, or both. An angiographic review may identify epicardial obstructive coronary artery disease that was overlooked on image acquisition or subtle abnormalities, such as distal small-vessel occlusion due to embolism or evidence of spontaneous coronary artery dissection. These findings would replace the working diagnosis of MINOCA with a more specific diagnosis.
We recommend that the referring physician and interventional cardiologist jointly review angiographic images in real time. This strategy allows the referring physician to most effectively transmit clinical history and imaging findings to the interventional cardiologist.
Depending on the findings, the interventional cardiologist can, in turn, decide to take the patient back to the catheterization laboratory for more tests (fractional flow reserve, instantaneous wave-free ratio, intravascular ultrasonography, optical coherence tomography, or coronary functional testing). This blended strategy allows for efficient use of the cardiac catheterization laboratory and avoids repeat invasive testing. Invasive coronary functional testing, as described in the Coronary Microvascular Angina (CorMicA) trial,12 is becoming more common but is not offered in all cardiac catheterization laboratories. Ideally, all these invasive tests should be done in 1 session so the patient does not have to go back, but this is not always possible.
Left ventricular functional assessment and cardiac magnetic resonance imaging for further characterization of the myocardium, in conjunction with angiographic findings, could similarly replace a MINOCA diagnosis with other mimics such as stress cardiomyopathy (takotsubo syndrome), nonischemic cardiomyopathy, or myocarditis. Mileva et al13 found that cardiac magnetic resonance imaging resulted in reclassifying 68% of patients with suspected MINOCA as actually having these mimics.
We note that left ventricular angiography (ventriculography), prominent in the AHA clinical algorithm11 and the earlier European Society of Cardiology paper,10 is less frequently used because high-quality transthoracic echocardiography and cardiac magnetic resonance imaging are widely available.
Green light: The patient has MINOCA; what is the cause?
If no alternative diagnosis is identified in the red and yellow algorithm steps, we can say the patient has MINOCA. At this point, additional diagnostic testing such as coronary functional assessment can be performed to find the specific underlying etiology for the MINOCA. In this idealized scenario, a thorough clinical history and coronary angiography precede imaging.
We applaud the AHA authors for promulgating a formalized evaluation pathway to search for an underlying etiology of MINOCA in each patient. How often the proposed algorithm will identify an underlying mechanism remains undefined. A clear mechanism of MINOCA was identified in only 25% of cases in the VIRGO registry, although patients did not routinely undergo cardiac magnetic resonance imaging.9
Once the diagnosis of MINOCA has been confirmed, subclassification into atherosclerotic or nonatherosclerotic disease can help determine the etiology and the management. As MINOCA is an umbrella term encompassing multiple discrete etiologies (Table 1),11 advanced cardiac imaging is essential in classifying the patient into the appropriate cause, which has important treatment and prognostic implications.
Myocardial infarction with nonobstructive coronary arteries (MINOCA): Potential mimics and causes
CASE CONTINUED: SEPTAL BRANCH OCCLUSION
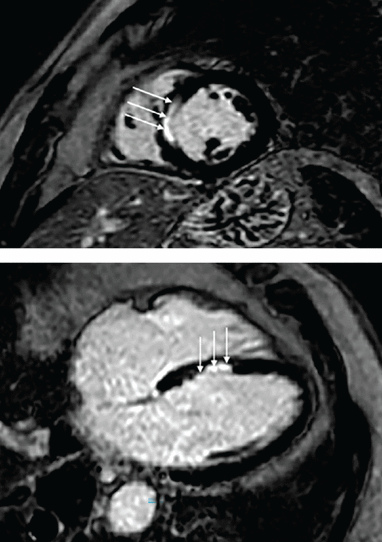
Our patient underwent cardiac magnetic resonance imaging, which demonstrated 50% thickness subendocardial enhancement at the mid and basilar anteroseptal wall on delayed imaging after gadolinium was administered, consistent with infarction in the territory of a septal perforator branch of the left anterior descending artery (Figure 4).
Delayed cardiac magnetic resonance imaging after administration of gadolinium (top, short-axis view; bottom, long-axis view), which demonstrates subendocardial enhancement (scar, white arrows) in the mid-septum amid otherwise normal-appearing left ventricular myocardium (black).
On further review of the angiographic images with the interventional cardiologist, a moderate stenosis of the proximal portion of the first septal perforator branch was noted. This stenosis was not clearly visualized on orthogonal or cranial views (Figure 5). Septal perforator branch occlusions are often caused by an upstream plaque rupture or more distant thromboembolism, such as a left atrial appendage thrombus. We did not think the patient had a ruptured plaque, as he had little plaque elsewhere in his coronary arteries, and a septal perforator branch is infrequently the site of a culprit lesion for an NSTEMI. Intracoronary imaging (optical coherence tomography or intravascular ultrasonography) is not feasible in such a small-caliber branch.
Cardiac magnetic resonance imaging. Anterior-posterior cranial view showing no epicardial obstructive coronary artery disease.
After close angiographic review and correlation with gadolinium-enhanced cardiac magnetic resonance imaging, the patient’s MINOCA was attributed to possible septal branch occlusion and spontaneous recanalization. The patient was treated with dual antiplatelet therapy, an oral beta-blocker, and a high-intensity statin. The patient did well and was discharged on the appropriate therapies.
OUTCOMES BY SUBTYPE UNCERTAIN
Because MINOCA has only recently been defined as a clinical entity, we have little data on long-term outcomes based on MINOCA subtype. Women with acute myocardial infarction due to spontaneous coronary artery dissection have a higher in-hospital mortality rate than those with acute myocardial infarction alone.14 The prognostic data from patients with thromboembolic MINOCA are predominantly derived from case reports. Studies of microvascular etiologies are difficult to interpret, as some did not differentiate atherosclerotic microvascular disease from other entities such as takotsubo cardiomyopathy.
As for the mimics of MINOCA, a prospective outcomes registry study showed little excess mortality over up to 10 years of follow-up for patients who were found to have myocarditis by cardiac magnetic resonance imaging, whereas the highest-risk subgroup was patients with cardiomyopathy (a grouping that included stress cardiomyopathy, hypertrophic cardiomyopathy, and dilated cardiomyopathy).15 Interestingly, cumulative mortality rates among patients with stress (takotsubo) cardiomyopathy were 3 times greater than those with alternative cardiomyopathy diagnoses (infiltrative, restrictive, hypertrophic, and other cardiomyopathies).
Among the patients with a confirmed diagnosis of MINOCA, independent predictors of death include advanced age and ST-segment elevation on presentation.16 The SWEDEHEART (Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies) registry16 found that independent predictors of major adverse cardiac events and cardiac death in MINOCA patients were similar to those of patients with myocardial infarction from obstructive epicardial coronary artery disease: advanced age, current smoking, diabetes, and reduced left ventricular ejection fraction.
MAJOR CAUSES OF MINOCA
Atherosclerotic cause: plaque disruption
Plaque disruption includes both plaque erosion and plaque rupture.
Plaque erosion is not well understood but is thought to involve apoptosis of endothelial cells secondary to loss of contact with the underlying extracellular matrix.17 Myocardial infarction after plaque erosion occurs when small thrombi form at the erosion site and subsequently embolize. It can, therefore, present without obstructive epicardial disease (< 50% stenosis) on angiography (MINOCA).
Plaque rupture, in contrast, occurs when there is acute disruption of the fibromuscular cap of the atherosclerotic plaque. In both cases, exposure of the subendothelium to the bloodstream triggers a thrombotic response that can lead to thrombotic coronary occlusion.17 Complete or near-complete thrombotic occlusion is more likely to be seen with plaque rupture in cases of myocardial infarction due to occluded coronary arteries. Coronary angiography displays the coronary lumen, rather than the vessel itself, and may not detect a small nonocclusive thrombus or thrombosis that has resolved with medical treatment before angiography.
Nonatherosclerotic causes
Spontaneous coronary artery dissection used to be thought to be rare, but it is a relatively common cause of sudden cardiac death and acute myocardial infarction in young women, occurring in 1% to 10% of women with acute coronary syndromes.14,16–19 Though it can present as MINOCA, the most common presentation is type 1 myocardial infarction, in which there is angiographic evidence of epicardial obstruction. However, many cases may be missed, and therefore the exact prevalence is unknown.20
Spontaneous coronary artery dissection can be overlooked on angiography, as the coronary arteries may appear minimally diseased. This is especially true in diffuse and smooth luminal narrowing (type 2 spontaneous coronary artery dissection), caused by an intramural hematoma compressing the lumen.
Vasculopathies and connective tissue disorders may predispose to spontaneous coronary artery dissection, a theory supported by the association between spontaneous coronary artery dissection and fibromuscular dysplasia.14
Spontaneous coronary artery dissection should be considered in any case of MINOCA, but it should be higher on the differential in women of childbearing age presenting with acute coronary syndrome and in patients without evident coronary artery plaque.
Coronary vasospasm. Coronary vasospasm can result from exogenous substances such as cocaine, amphetamines, and certain medications (5-fluorouracil, selective serotonin agonists such as sumatriptan) or intrinsic smooth-muscle hyperreactivity. Coronary artery spasm was initially described by Prinzmetal et al21 in patients with nonobstructive atherosclerotic disease. Classically, transient vasospastic episodes can produce ST elevation, but some episodes can be associated with ST depression. Angina is typical, though frequently not exertional. Prolonged episodes of vasospasm can lead to myocardial ischemia and subsequent MINOCA.
Coronary microvascular disease. Though an in-depth discussion is beyond the scope of this article, coronary microvascular disease (or microvascular dysfunction) is common in patients with MINOCA.22 Microvascular disease involves arteries less than 0.3 mm in diameter, which are not adequately visualized on coronary angiography. It can manifest as vasospasm, as discussed above, or microvascular angina, which manifests as endothelial dysfunction without observed spasm of the larger vessels. Impaired microvascular function can exacerbate flow restrictions in the epicardial vessels and play an important role in MINOCA.
Cardiac magnetic resonance imaging and positron-emission tomography can help assess for microvascular disease, but the gold standard for diagnosis is functional coronary angiography, as described in the CorMICA trial.12 If vasospasm or microvascular angina is diagnosed on functional coronary angiography, specific treatment can be given, and noncardiac chest pain can be excluded. Recent European Society of Cardiology guidelines for managing acute coronary syndrome23 suggest that these tests can be performed at the time of initial angiography if no obstructive coronary artery disease is identified (ie, in MINOCA), but in practice they are not yet widely available. Patients may need to be referred to a tertiary care center or repeat angiography after microvascular dysfunction is suggested on noninvasive imaging.
Coronary thromboembolic disease. Coronary thromboembolism can result in MINOCA if partial or complete lysis occurs before angiography. Embolism can arise from a remote location, such as the left atrial appendage in the setting of atrial fibrillation, or it could result from downstream embolization in acute coronary syndrome. Anecdotally, “local” embolism is more likely to resolve with medical therapy than “remote” thrombi, given the lack of thrombin cross-linking in the former. Coronary thrombi or emboli—as with any other cause of MINOCA—can occur in the presence or absence of acquired or inherited hypercoagulable states.
CLINICAL WORKUP OF MINOCA: WHAT’S NEW?
A diagnostic algorithm. We endorse the AHA traffic light algorithm for the diagnosis of MINOCA (Figure 3).11 In this paradigm, red excludes nonischemic etiologies, yellow suggests slowing down to evaluate for alternate diagnoses that can mimic MINOCA, and green suggests a confirmed diagnosis of MINOCA. If there is no emergent or urgent indication for coronary angiography, we suggest carefully considering the clinical picture to exclude common but nonischemic causes of troponin elevation, such as sepsis, end-stage renal disease, cardiac contusion in the setting of trauma, and pulmonary embolism.
If acute myocardial infarction remains the most likely diagnosis, it is reasonable to proceed to coronary angiography. For patients without at least 50% epicardial coronary artery stenosis, it is important to review the coronary angiography with the interventional cardiologist to exclude overlooked disease, namely distal small-vessel obstructive disease, spontaneous coronary artery dissection, and coronary emboli or thrombus (yellow section of Figure 3).
Intracoronary imaging with intravascular ultrasonography or optical coherence tomography should be considered in patients with MINOCA and less than 50% stenosis on coronary angiography. Intravascular ultrasonography can often identify plaque rupture and atherosclerosis.
In one study, plaque rupture or erosion was diagnosed by intravascular ultrasonography in 16 (42%) of 42 women with MINOCA.24 Several other studies reported that plaque rupture is visualized on intravascular ultrasonography in roughly one-third of patients with MINOCA.3,4 The number of patients with MINOCA who have plaque erosion is unknown, as intravascular ultrasonography does not reliably detect it.24,25 Further, plaque erosion is more common in women and younger patients, consistent with the overall demographics of MINOCA.26
Optical coherence tomography is not available in all centers, but it has superior spatial resolution and can be used to evaluate patients for plaque erosion. Although they can be helpful, intravascular ultrasonography and optical coherence tomography are used sparingly to diagnose spontaneous coronary artery dissection, as they may propagate the dissection.27,28
Invasive functional assessment of the epicardial vessels by measuring the instantaneous wave-free ratio or fractional flow reserve can be helpful in determining physiologically significant disease that may be overlooked on angiography alone.11
Stress imaging. If angiography is completed without physiologic assessment using fractional flow reserve or instantaneous wave-free ratio (or functional coronary angiography), ischemia can be detected on functional stress testing using nuclear (single-photon emission computed tomography, positron-emission tomography) or magnetic resonance stress imaging. Stress cardiac magnetic resonance imaging can be helpful in reaching a specific diagnosis. Alternatively, positron-emission tomography with computed tomography can detect ischemia and allows for the assessment of microvascular function using the myocardial blood flow ratio.11
Cardiac magnetic resonance imaging. If a careful review of the coronary angiography films and adjunctive intracoronary imaging are not revealing, cardiac magnetic resonance imaging is a key diagnostic tool. It is the imaging modality of choice for MINOCA in both the European10 and the AHA guidelines.11 It can provide evidence to support a diagnosis of (type 1) myocardial infarction, as in our patient, or an alternate diagnosis, such as myocarditis, takotsubo syndrome, or cardiomyopathy. On the other hand, it has multiple barriers to routine use, including availability, cost, potential increased hospital length of stay, patient discomfort due to claustrophobia, and inability to use gadolinium in patients with impaired renal function.
Early studies of cardiac magnetic resonance imaging in MINOCA suggested it has a lower diagnostic yield if troponin levels are lower.29 The European Society of Cardiology position paper10 on MINOCA suggests it has a low diagnostic yield when the troponin is less than 100 times the upper limit of normal. A report by Dastidar et al30 suggests that definitive diagnosis can be achieved after cardiac magnetic resonance imaging in three-quarters of MINOCA cases. We would expect that the diagnostic yield may be lower in a less-selected patient population or if patients with lower-level troponin elevation are routinely included—the mean troponin elevation was 14 times the upper limit of normal in the subset with normal cardiac magnetic resonance imaging compared with 48 times the upper limit of normal in the MINOCA diagnosis group.30
Provocative testing for vasospasm. The diagnosis of vasospastic MINOCA requires a demonstration of coronary artery spasm with provocative testing.31 For patients presenting with chronic, episodic, non-exertional angina, in whom coronary artery spasm is considered likely and evaluation has been unrevealing, provocative testing may be appropriate. The safety of intracoronary acetylcholine has been demonstrated in stable patients, with death and major adverse cardiac event rates no higher than those of coronary angiography.13,14,16 Typically, diagnosis relies on clinical assessment and electrocardiographic findings (standard or ambulatory monitoring), with provocation tests only occasionally done.
Empiric treatment of presumed vasospastic MINOCA with calcium-channel blockers or long-acting nitrates is low-risk. It has been shown to reduce the recurrence of symptoms.32
Coagulation studies. In patients with unprovoked coronary thromboembolism and MINOCA, without evidence of paroxysmal atrial fibrillation, evaluation for a hypercoagulable state can be considered in conjunction with a hematologist in the outpatient setting.
TREATMENT
As MINOCA is a recently recognized clinical syndrome that occurs secondary to disparate underlying diagnoses, the evidence to support specific treatments for it is both limited and heterogeneous. Treatment of all patients with MINOCA should include and emphasize lifestyle management, especially in those with diffuse nonobstructive atherosclerosis (< 50%), microvascular disease, or a supply-demand mismatch.
Most cases of MINOCA are due to causes that are common, and therefore, guideline-directed therapies for secondary prevention of atherosclerotic cardiovascular disease may be indicated. Among patients with MINOCA enrolled in the SWEDEHEART registry,16 treatment with statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers was associated with a reduction in major adverse cardiac events during a 4-year follow-up. There was a trend toward improved outcomes in patients treated with beta-blockers, which did not reach statistical significance. The use of dual antiplatelet therapy was not associated with improved outcomes.
That said, medical therapies should be tailored to the underlying etiology. For example, patients with diffuse nonobstructive disease or microvascular disease are more likely to benefit from statin therapy, whereas its routine use is not recommended in patients with spontaneous coronary artery dissection. In patients with spontaneous coronary artery dissection, a conservative approach is recommended to avoid instrumentation of the artery and propagating the dissection plane. The use of calcium channel blockers in those with coronary spasm has been shown to reduce symptoms, and there is evidence that anticoagulation may be appropriate for the prevention of thromboembolic disease.12,33,34
We hope that with a standardized diagnostic pathway that regularly includes cardiac magnetic resonance imaging, as suggested by the AHA scientific statement,11 most patients with MINOCA will receive a specific diagnosis to guide therapy. Routine assessment may provide a specific diagnosis in many cases of MINOCA, and we may be able to avoid unnecessary treatments in up to three-quarters of cases. Moreover, patients with a normal cardiac magnetic resonance imaging result may not require any medical therapy. Collaborative studies of MINOCA and other less-common cardiac diseases, including spontaneous coronary artery dissection and stress cardiomyopathy, may be able to identify a more homogeneous patient population to define optimal treatment strategies.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.

Clicking the link below will connect you to begin the credit-claiming process for CME and MOC. After clicking on the link, scroll to the bottom of the page and click on “Complete the CME/MOC Process.” You will need your myCME login information to access this.