ABSTRACT
An estimated 1.2 million people in the United States have human immunodeficiency virus (HIV) infection per US Centers for Disease Control and Prevention 2021 data. The highest risk of HIV transmission occurs during injection drug use with needle sharing and during sexual activity, most significantly in condomless, receptive anal intercourse. Preexposure prophylaxis (PrEP) for the prevention of HIV infection is part of a larger biobehavioral strategy that uses antiretroviral medication, an oral formulation taken daily or during anticipated exposure events, or an injectable formulation administered every 8 weeks. PrEP consists of 3 possible regimens: emtricitabine/tenofovir disoproxil fumarate, emtricitabine/tenofovir alafenamide, or injectable cabotegravir. Primary care clinicians are strategically positioned to provide PrEP education and access.
The 3 available PrEP regimens are emtricitabine/tenofovir disoproxil fumarate, emtricitabine/tenofovir alafenamide, and injectable cabotegravir.
The highest risk of HIV transmission occurs during injection drug use with needle sharing and during sexual activity, most significantly in condomless receptive anal intercourse.
Conducting a sexual history is a first step in identifying behaviors that may place patients at risk for, or protect them from, exposure to HIV.
The US Centers for Disease Control and Prevention 2021 data estimate that 1.2 million people in the United States have human immunodeficiency virus (HIV) infection,1,2 and that 766,000 people have died from complications of HIV infection between the first report of HIV in 1981 and 2019.2,3 Despite a downturn in new cases of HIV infection in the United States over the past decade, 30,635 new infections were reported in 2020.2,4 While this is a 17% decrease from 2019, 36,136 new infections were reported in 2021, an 18% increase,5 possibly owing to healthcare disruption during the COVID-19 pandemic.1,4 Of these new cases, about 80% are in adolescents (age ≥ 13) and adult men, and 68% are in men who have sex with men (MSM).2,4
Social disparity contributes to the epidemiology of HIV infection.1,2,5,6 Black or Hispanic persons, transgender individuals (specifically transgender women), and persons who inject drugs are disproportionately affected compared with the general population.2,5,6 The highest risk of HIV acquisition occurs during injection drug use with needle sharing and during sexual activity, most significantly in condomless receptive anal intercourse. The likelihood that a specific activity will result in HIV infection is related to the detectable viral load of a partner with HIV, compounded by concurrent inflammatory sexually transmitted infections.2,7
Preexposure prophylaxis (PrEP) for the prevention of HIV infection is part of an effective biobehavioral intervention that includes antiretroviral medication taken daily or during anticipated exposure events.6,8 Currently, there are 3 PrEP regimens available: emtricitabine/tenofovir disoproxil fumarate, emtricitabine/tenofovir alafenamide, and injectable cabotegravir. In 2021 it was estimated that only 30% of the 1.2 million people who could benefit from PrEP were prescribed this therapy.9 Although evidence does demonstrate occurrence of drug-resistant HIV infection during PrEP therapy, the incidence is rare. Most resistant cases were reported when treatment was started during an undiagnosed acute HIV infection or during inconsistent PrEP use.10 When adherence to PrEP regimens is at least 70%, the approximate reduction of risk of HIV infection is 75% (approximate number needed to treat = 33).11
Primary care clinicians are positioned to provide PrEP education and access.12
PrEP IN PRACTICE
Screening
The US Preventive Services Task Force recommends screening all adults and adolescents age 15 or older for HIV.13 All sexually active persons should be informed about PrEP for the prevention of HIV infection.14 PrEP is recommended if behaviors indicate risk of HIV acquisition, as it is a safe and effective prevention strategy.15
Patients often do not disclose stigmatized sexual or substance use behaviors to clinicians, especially when not asked.14 Routinely taking a sexual history creates an opportunity to provide appropriate sexual healthcare.15,16 Often this history is not completed due to urgency of other medical conditions, time constraints, clinician discomfort and bias of sexual practice, and lack of experience and familiarity in discussing sexual healthcare topics.12,14,17–20 Using a sexual history framework based on cultural humility, context, and open conversation helps to develop rapport and trust, especially in a sexual and gender minority population. A guided discussion of sexual history increases the effectiveness of the conversation and may decrease the associated anxiety of both patient and clinician.21
Conducting a sexual history is a first step in identifying behaviors that may place patients at risk for, or protect them from, exposure to HIV.2,13 Elements of a complete history include sex assigned at birth with organ inventory; gender identity; sexual orientation, practices, and partners; transactional or commercial sex work; sexually transmitted infections; protective practices; intention of pregnancy; and prevention methods (Table 1).2,8,14,21
Suggested questions for obtaining a sexual history
Screening for alcohol use disorder, use of noninjecting illicit drugs, and receptive sharing of syringes and injection equipment of illicit drugs also helps determine risk of HIV acquisition and whether PrEP therapy may be considered.2,14 Further, referrals should be placed for substance-use support and harm-reduction interventions.6,14
Indications for PrEP
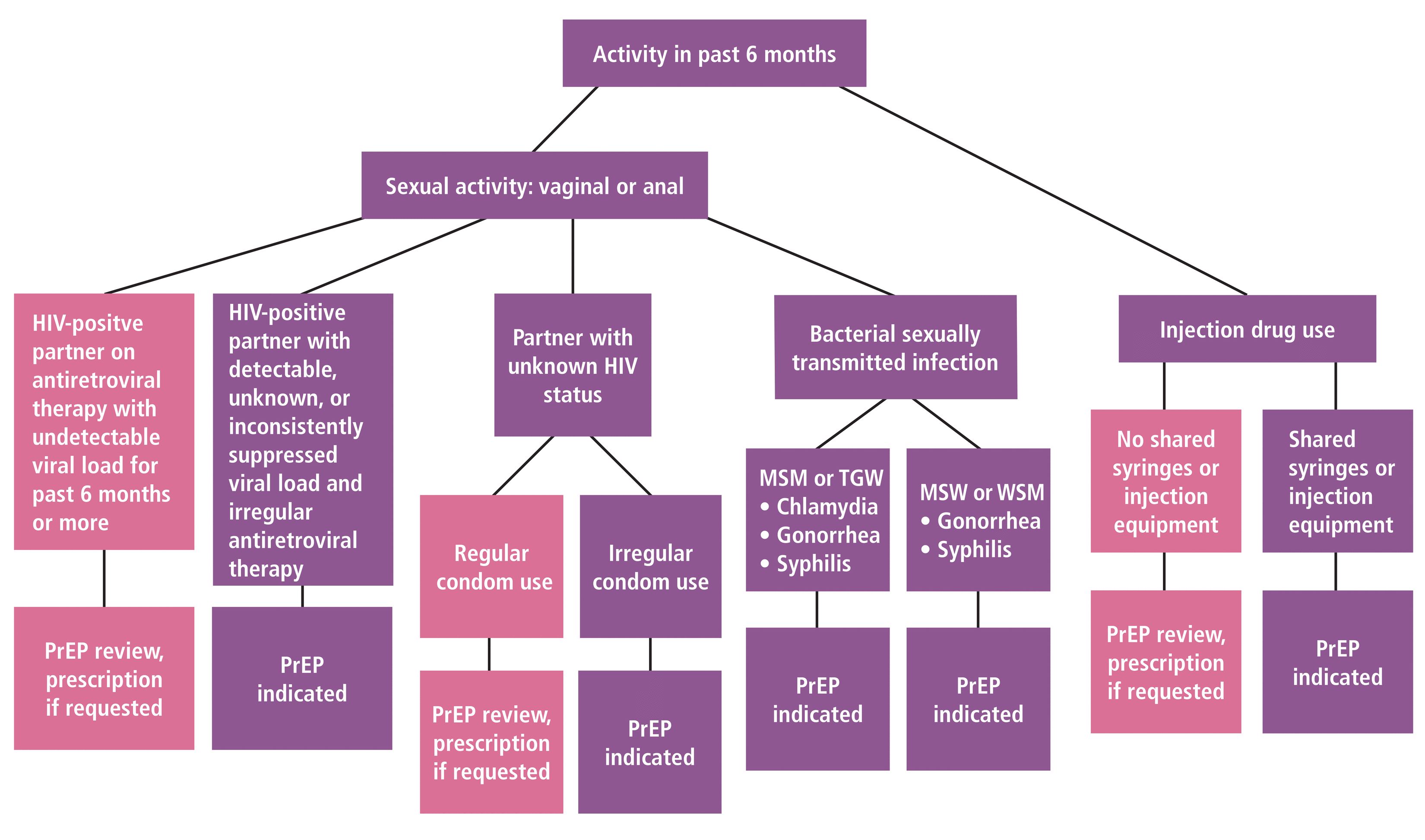
Indication for PrEP can be assessed using the strategy illustrated in Figure 1.2,6,14,22–25 Vaginal or anal sexual activity or history of injection drug use with shared syringes or injection equipment in the past 6 months directs care. If no activity is disclosed, information about PrEP should be reviewed. Regardless of disclosed or identified risk behaviors, any patient who requests PrEP should be offered this therapy.2,6,14
Considerations for initiation of preexposure prophylaxis.
HIV = human immunodeficiency virus; MSM = men who have sex with men; MSW = men who have sex with women; PrEP = preexposure prophylaxis; TGW = transgender women; WSM = women who have sex with men
Sexual activity with an HIV-positive partner receiving antiretroviral therapy with an undetectable viral load (ie, less than 200 copies/mL) for at least the previous 6 months prompts PrEP review and prescription, if requested. This is noted in the 2016 Prevention Access Campaign’s health equity initiative, using the slogan Undetectable = Untransmittable or U = U, signifying that an individual with an undetectable viral load cannot sexually transmit the virus to others.22,26 PrEP is indicated in the following scenarios:
A partner’s viral load is detectable, not known, or inconsistently suppressed, or if use of antiretroviral therapy is irregular
The patient is sexually active with any partner without knowing the partner’s HIV status, or with irregular condom use; consistent condom use directs PrEP review and prescription, if requested
The patient has had a bacterial sexually transmitted infection in the past 6 months, specifically chlamydia, gonorrhea, or syphilis in MSM and transgender women, and gonorrhea or syphilis in men who have sex with women and women who have sex with men.2
Although there is no indication for PrEP for reducing HIV risk from injection drug use with shared syringes or injection equipment within the past 6 months, guidelines note that patients who engage in such activity likely benefit from these medications.2 If patients do not endorse drug use activity or sharing of items, PrEP should be reviewed and prescribed if requested.
TESTING GUIDELINES
HIV testing
The US Centers for Disease Control and Prevention and the US Preventive Services Task Force recommend that MSM, persons who inject drugs, patients with an HIV-positive sexual partner, and persons at substantial risk of HIV transmission have annual HIV testing.2,14 If additional risk factors are present, testing every 3 to 6 months is recommended.
Documented, confirmed negative HIV-testing results within the week are required before starting PrEP therapy, and HIV testing should be repeated at intervals after PrEP initiation.2,14 Testing options include an HIV antigen-antibody assay, viral load and nucleic acid (or RNA) laboratory testing, or US Food and Drug Administration (FDA)–approved point-of-care fingerstick antigen-antibody blood tests.2,14 If point-of-care testing is used, a laboratory antigen-antibody test should always be ordered with baseline laboratory screening, as point-of-care testing has lower sensitivity. This practice increases detection of an unrecognized acute infection.2,14
Testing for bacterial sexually transmitted infections
Testing for gonorrhea, chlamydia, and syphilis is recommended at least annually.2,6,14 For gonorrhea and chlamydia, 3-site testing of self-collected urine or vaginal specimens, pharyngeal swabbing, or rectal swabbing is recommended at PrEP initiation and for MSM at quarterly visits.2,14 Semiannual gonorrhea and annual chlamydia testing is recommended for cisgender women. Chlamydia infection, in contrast to syphilis and gonorrhea, does not have a strong correlation with risk of HIV transmission and may be screened for less frequently.
Gonorrhea or chlamydia infection in men who have sex with women and women who have sex with men warrants consideration of expedited partner therapy. Based on limited data regarding expedited partner therapy in MSM and the incidence of other bacterial sexually transmitted coinfections, shared clinical decision-making regarding expedited partner therapy is recommended.2,14 In cases of syphilis or HIV diagnoses, referral for partner services is advised.
Syphilis screening, commonly performed with a rapid plasma reagin test, is recommended at least annually for those at increased risk of infection based on high-risk sexual and injection drug use behaviors and during each pregnancy.2,6 Periodic testing may be performed every 3 to 6 months and as requested.
Laboratory testing
Estimated creatinine clearance rate (mL/min) calculation and serum creatinine testing should be done for patients on oral PrEP because decreased renal function is a potential safety issue.2,27 Renal function should be assessed every 6 to 12 months.
Tenofovir and emtricitabine are used to treat chronic hepatitis B virus infection. Patients with chronic hepatitis B who discontinue PrEP may experience a significant hepatitis flare. Hepatitis B virus testing allows for this risk to be considered and patients counseled accordingly if screening is positive. Screening for hepatitis B virus infection is performed by measuring hepatitis B surface antigen, hepatitis B surface antibody, and total antibody to hepatitis B core antigen.2,27 Ideally, this triple-panel screening should be performed before PrEP is started, but screening should not delay therapy. Initiating PrEP in patients with chronic hepatitis B virus infection should be done in consultation with an expert in hepatitis B virus treatment.2,27
Hepatitis C screening is recommended at least once in a lifetime for all adults age 18 or older and during each pregnancy.28 Annual and periodic testing may be performed for persons with ongoing high-risk behaviors, including persons who inject drugs or participate in receptive condomless anal sex, or as requested.
Annual lipid panel monitoring should be performed for patients on emtricitabine/tenofovir alafenamide as clinical trials have shown greater weight gain and elevation in triglyceride levels in MSM and transgender women taking this medication than in those taking emtricitabine/tenofovir disoproxil fumarate.2,14 This increased risk may be considered when treating patients with preexisting cardiovascular risk.
Dual-emission x-ray absorptiometry scans should be considered in patients with a history of osteopenia or pathologic bone fracture.22
Recommended testing for patients starting and taking PrEP therapy is summarized in Table 2.2,6,14,22–25
Testing in patients prescribed preexposure prophylaxis for HIV prevention
PRESCRIBING: ORAL MEDICATION
Two oral PrEP medications, both 2-drug combinations of HIV-1 nucleoside analogue reverse transcriptase inhibitors, are approved by the FDA, and have a Grade A recommendation from the US Preventive Services Task Force (Table 3).2,6,14,22–25,29 In men at risk for sexual exposure to HIV, oral PrEP medications have high efficacy (up to 99% when taken as prescribed) and good safety profiles.14,22,23,30 PrEP is not approved for expedited partner therapy.
Preexposure prophylaxis prescribing, safety, and other considerations
In 2021 the US Department of Health and Human Services established that some federal health programs and most commercial insurers must provide oral PrEP medication, indicated laboratory tests, and clinic visits with no out-of-pocket cost to patients.14 However, navigating insurance coverage of PrEP can be challenging. Prior authorizations are commonly required, and copay amounts vary. Employing diagnosis codes, such as those suggested by the HIV Medicine Association (Table 4), is recommended.31 Use of thoughtful, nonstigmatizing nomenclature provides additional rapport and trust between clinician and patient.
Commonly used preexposure prophylaxis diagnosis codes
Daily Regimens
Emtricitabine/tenofovir disoproxil fumarate is a fixed-dose combination (200/300 mg) daily medication for use in healthy (estimated creatinine clearance rate > 60 mL/minute) cisgender and transgender adults and adolescents (≥ 35 kg) at risk of acquiring HIV infection.14,22,32 Emtricitabine/tenofovir disoproxil fumarate is available in generic and brand name forms. It is FDA-approved for use in MSM, transgender women, and cisgender women, including those seeking to conceive or who are pregnant or breastfeeding. Data are not available regarding transgender men, genderqueer, or nonbinary individuals who become pregnant and deliver while taking PrEP. Concentrations of emtricitabine/tenofovir disoproxil fumarate may be increased with medications that reduce renal function (antivirals, aminoglycosides, and high-dose nonsteroidal anti-inflammatory drugs).14,22 In patients taking feminizing hormones, studies report potential reduction in rectal tissue concentration, but the effect on efficacy is unclear.14
Emtricitabine/tenofovir alafenamide is a fixed-dose combination (200 mg/25 mg) daily medication for use in men and transgender women at sexual risk for acquiring HIV infection.14,23 It is available only in brand formulation and is approved for use in persons with an estimated creatinine clearance rate of at least 30 mL/minute.14,23 Emtricitabine/tenofovir alafenamide is not an FDA-approved PrEP medication for use by women at risk of acquiring HIV through receptive vaginal sex, as research has not been reported in this population.2,14,23,24 Several medications may decrease the concentration of emtricitabine/tenofovir alafenamide and should not be coadministered, including St. John’s wort and the antibiotics rifabutin and rifapentine.
The time from initiation of daily oral PrEP to maximum protection against HIV infection is not clear. Although pharmacokinetic studies show that intracellular concentration occurs after 7 to 20 days of daily oral dosing, depending on tissue type, exactly when maximal tissue protection takes place is not known.2,14 Use of condoms is recommended during this time; in fact, promoting consistent use of condoms is a component of successful PrEP treatment.2,9,14
The most-reported side effects with oral PrEP include headache, nausea, diarrhea, and weight change.2,22,23 Barriers to adherence to oral PrEP should be reviewed.2,6,20,33 Daily alarms and routines have been found to be helpful, as has acknowledging the incidence of occasional missed doses.
On-demand PrEP
On-demand PrEP, or the 2-1-1 regimen (also called event-driven or intermittent), times dosing of oral emtricitabine/tenofovir disoproxil fumarate in relation to sexual intercourse events.9,14 The regimen consists of 2 pills taken 2 to 24 hours before sex, preferably closer to 24 hours, 1 pill taken 24 hours after the initial 2-pill dose, and 1 pill taken 24 hours after this. Should sex occur on the consecutive day after the 2-1-1 doses are completed, an additional 1 pill per day should be taken until 48 hours after the last sexual event. If there are fewer than 7 days until the next sexual event, the patient should resume taking 1 pill daily. If 7 days or more have passed, the patient should restart 2-1-1 dosing.
Negative HIV testing is required before starting on-demand PrEP, and a prescription should be for no more than 30 days, with follow-up testing required for continuation of medication.6,14 Testing for bacterial sexually transmitted infections is also recommended with on-demand dosing, at intervals similar to the testing intervals for daily dosing.
The 2-1-1 regimen is advantageous for use in cisgender men who have sex less than once per week and can take the initial dose at least 2 hours before anticipated sex.6,19 On-demand dosing should be taken for every episode of sexual activity, not selectively. Although the 2-1-1 regimen is not FDA-approved for MSM, studies have shown that it is effective for HIV prevention in MSM, and the International Antiviral (formerly AIDS) Society-USA panel has recommended 2-1-1 dosing as an optional, off-label alternative to daily dosing for MSM.8,34
Same-day prescribing
Initiation of PrEP therapy may be delayed because of the multiple healthcare visits required to receive a prescription; same-day prescribing can help mitigate this barrier to starting PrEP for those at substantial risk of acquiring HIV infection.6,8,35 Same-day prescribing can only be offered in clinics that provide HIV point-of-care testing or provide same-day results for HIV and creatinine laboratory testing. Ideally, screening for sexually transmitted infections should also be performed at this time. Clinics considering same-day PrEP prescribing must be able to provide rapid-result follow-up, follow-up appointment scheduling, and patient navigation support for prescription payment assistance.8
Eliminating serial visits to healthcare facilities for initiation of PrEP may benefit patients who have confidently made the decision to start treatment, can complete a blood draw, and have no renal or other medical conditions that may decrease adherence with PrEP therapy. Reliable contact information, having the ability to acquire prescribed medication, and not having had recent HIV exposure are required.20 Those with recent HIV exposure without acute symptoms should be evaluated for postexposure prophylaxis (outside the scope of this review).
Follow-up
Patients on oral PrEP should follow-up every 3 months for HIV testing, screening for sexually transmitted infections, and support for medication adherence and risk-reducing behaviors.32 When starting PrEP therapy, the option of a 30-day prescription and 1-month follow-up for repeat HIV testing and counseling may be pursued. Prescriptions for daily PrEP medication should not exceed 90 days.14
The COVID-19 pandemic led to disruptions in clinical care services, including HIV treatment and PrEP therapy, and shortages of HIV testing reagent and materials,7,29 but it also led to an increase in telehealth services. Telehealth has become a prominent healthcare delivery tool that can help patients hesitant to access in-person clinical services. It has led to adaptations in starting and continuing oral PrEP treatment, overcoming potential barriers to access.1 Additionally, telemedicine may reduce potential stigma associated with initiation and continuation of PrEP therapy, reduce transportation time and cost, and reduce the financial penalty of loss of work hours for patients.7 All told, mitigation of these barriers could substantially improve access to PrEP therapy. Testing for HIV infection and sexually transmitted infections as well as renal function testing can be done virtually by using at-home testing or scheduling with available laboratories.29
PRESCRIBING: INJECTABLE MEDICATION
One FDA-approved injectable PrEP medication, an HIV-1 integrase strand transfer inhibitor, is now available.25 The current recommendation from the US Preventive Services Task Force for PrEP does not apply to injectable formulations. Cabotegravir injections may be considered for cisgender men, cisgender women, and transgender women (> 35 kg) who have difficulty taking daily oral PrEP medications, have renal impairment, or prefer injectable therapy.6,24,26 Oral cabotegravir 30 mg daily is available for an optional 4-week lead-in before starting injectable therapy.25 Nonadherence to the lead-in course creates a potential vulnerability for HIV acquisition.6 Patients taking daily oral PrEP can start cabotegravir injection if they test negative for HIV infection.
Cabotegravir is administered as a 600-mg gluteal intramuscular injection.6,29,36 Time from initiation of cabotegravir to maximum protection against HIV infection is not yet known.
Follow-up
A negative HIV test is required within the week before starting cabotegravir and during maintenance dosing.36 The next dose after initiation is administered 1 month later, and thereafter maintenance doses are scheduled every 2 months.35 Screening for sexually transmitted infections is recommended at regular intervals. If injections are more than 7 days late, an oral PrEP bridge is recommended to maintain protection until the next injection. With any injection more than 8 weeks late, a reloading dose schedule with 4-week intervals should be followed. HIV-1 RNA testing should be performed at the time of oral PrEP bridge and restart of injections.6,14,37
DISCONTINUATION OF PrEP
Situational life changes, nonadherence to daily dosing, nonadherence to follow-up or laboratory testing, intolerance to medication, or acquisition of HIV infection may prompt discontinuation of PrEP. HIV status and the reason for discontinuation should be documented. HIV infection protection wanes in 7 to 10 days after oral PrEP is discontinued, and patients with chronic hepatitis B should be monitored for flares.37 Methods to reduce risk for HIV acquisition should be discussed, as should indications for postexposure prophylaxis. Changes prompting a restart of PrEP, which follow the same guidelines as original initiation, need to be documented.2,14,22,23,36
Discontinuation of cabotegravir includes a tail period as protective levels decline. There is a possibility of false-negative HIV testing during this time and risk of developing drug-resistant HIV infection.14 HIV testing should continue every 3 months for 12 months. If risk remains and PrEP continues to be indicated, daily oral PrEP should be started within 8 weeks of the last injection.6,14,25
PREVENTIVE HEALTHCARE OPPORTUNITIES
Providing PrEP opens opportunities to provide preventive healthcare during the required frequent visits. Mental health, nicotine, alcohol and drug use, and intimate partner violence screenings should be conducted. Anatomy-specific screenings for cervical, chest or breast, colorectal, and prostate cancers are recommended per the US Preventive Services Task Force guidelines.38 Anal cancer screening is recommended per the International Anal Neoplasia Society consensus guidelines.39 The Advisory Committee on Immunization Practices recommends discussing immunizations against hepatitis A, hepatitis B, mpox (formerly monkeypox) virus, human papillomavirus, meningitis B, influenza, COVID-19, pneumonia, respiratory syncytial virus, and shingles.40
PrEP IN PRIMARY CARE
Primary care clinicians are strategically positioned to deliver sexual healthcare, including PrEP therapy, to their communities.8,15 The impact of local and accessible continuity of care cannot be overstated as the benefit of PrEP therapy is significant and far reaching. Primary care clinicians who can confidently conduct a thorough sexual history and know the benefits and prescribing practice of PrEP may significantly help reduce the incidence of new HIV infection. Education resources for clinicians and patients are listed in Table 5.
Preexposure prophylaxis education and prescribing resources
DISCLOSURES
The author reports no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.