A 47-year-old woman was admitted to the emergency department because she had been struggling to stand up for the previous 10 days. She did not have difficulty breathing or history of similar episodes of weakness.
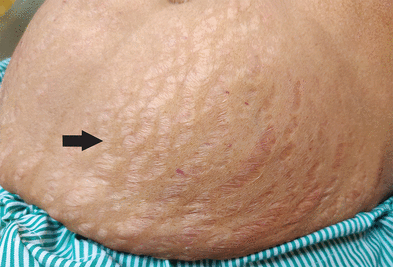
Physical examination showed high blood pressure (160/90 mm Hg) and the presence of wide purple striae on the abdomen (Figure 1). Marked hyperpigmentation was also noted on the knuckles (Figure 2).
Striae on the patient’s abdomen (arrow).
Hyperpigmentation of the patient’s knuckles (arrows).
Review of medical records revealed persistent severe hypokalemia, with a potassium of 2.3 mmol/L (reference range 3.5–5.1 mmol/L); however, the patient had no history of recent diarrhea, vomiting, or diuretic use.
A workup was performed, and the laboratory test results are listed in Table 1. The patient’s transtubular potassium gradient and 24-hour urine potassium were 9 and 117 mmol, respectively, suggesting inappropriate renal loss of potassium. The patient’s serum cortisol value after an overnight 1-mg dexamethasone suppression test was 48.0 μg/dL, suggesting endogenous cortisol excess, while the cortisol value after a high-dose (8 mg) dexamethasone suppression test was 42.7 μg/dL, indicating the presence of a nonpituitary adrenocorticotropic hormone (ACTH)–secreting tumor.
Patient’s laboratory test results
A provisional diagnosis of ACTH-dependent Cushing syndrome was made. Dynamic magnetic resonance imaging of the pituitary gland was performed and showed normal results. Contrast-enhanced computed tomography of the chest and abdomen revealed bilateral adrenal enlargement. Gallium-68 dotatate positron emission tomography-computed tomography to localize the source of ectopic ACTH secretion was negative. Therefore, a diagnosis of occult ectopic ACTH-secreting Cushing syndrome was made.
The patient was started on potassium supplementation, spironolactone 100 mg daily, and ketoconazole 400 mg daily, which was titrated to maintain potassium in the near-normal range.1 Subcutaneous insulin was also administered for high plasma glucose, and oral trimethoprim-sulfamethoxazole was started as prophylaxis against Pneumocystis jirovecii infection because of the patient’s markedly high cortisol level.2 She showed symptomatic improvement.
The patient refused to undergo bilateral adrenalectomy, was discharged on ketoconazole, and is currently under regular follow-up care.
DIFFERENTIAL DIAGNOSIS OF HYPOKALEMIA
Hypokalemia, a common electrolyte abnormality in hospitalized patients, can occur because of decreased oral intake, transcellular shift, or renal or extrarenal loss of potassium.3 Decreased oral intake is seen in states of starvation, whereas transcellular shift can occur because of alterations in acid-base homeostasis such as metabolic alkalosis; release of insulin, thyroid hormone, or catecholamines; or hypokalemic periodic paralysis. Extrarenal loss is predominantly caused by severe diarrhea or excessive sweating. The loss of gastrointestinal contents by vomiting causes secondary hyperaldosteronism and, subsequently, renal loss of potassium.
Other causes of renal loss include states of primary mineralocorticoid or cortisol excess and augmented distal urine flow that commonly occurs with diuretic therapy and salt-wasting nephropathies. Aldosterone activates the epithelial sodium channels in principal cells, increasing potassium excretion, which causes hypokalemic alkalosis. At the same time, the presence of excess cortisol in Cushing syndrome, as seen in our patient, overwhelms the 11-beta hydroxysteroid dehydrogenase enzyme in distal tubules and activates mineralocorticoid receptors, resulting in hypertension and hypokalemic alkalosis.3
Laboratory testing pointed to renal loss of potassium due to hypercortisolism resulting from a nonpituitary ACTH-secreting tumor as the cause of hypokalemia in this patient. A transtubular potassium gradient (calculated by dividing the urine potassium:plasma potassium ratio by the urine osmolality:plasma osmolality ratio) less than 2 and 24-hour urine potassium less than 15 mmol in the setting of hypokalemia suggests appropriate renal conservation of potassium3; both values were elevated for this patient.
Further, the patient’s cortisol level was not suppressed in either the overnight or high-dose suppression test. In healthy individuals, serum cortisol after an overnight 1-mg dexamethasone suppression test is suppressed to less than 2.0 μg/dL, but patients with endogenous Cushing syndrome are resistant to this suppression.1 After a high-dose (8 mg) dexamethasone suppression test, greater than 50% suppression of cortisol from baseline reflects a reduction in ACTH secretion in response to high-dose glucocorticoid, indicating Cushing disease (ie, Cushing syndrome caused by pituitary hypersecretion of ACTH). Less than 50% or no suppression occurs in ectopic ACTH-secreting Cushing syndrome, as nonpituitary ACTH-secreting tumors typically do not respond to glucocorticoid negative feedback.1
DIAGNOSIS AND TREATMENT
The diagnostic approach in a patient with hypokalemia includes a careful history to rule out common offending medications, such as diuretics and laxatives, and the presence of vomiting or diarrhea. A physical examination should be done to identify specific signs of a particular disease such as Cushing syndrome and thyrotoxicosis. Urine electrolyte measurements further help with diagnosis. A metabolic panel, including serum bicarbonate, along with an assessment of volume status identifies patients with renal potassium loss. The presence of a non–anion-gap metabolic acidosis suggests renal tubular acidosis.3 Metabolic alkalosis indicates either Bartter syndrome, Gitelman syndrome, vomiting, or diuretic use, whereas the presence of hypertension points to hyperaldosteronism or Cushing syndrome.4
Treatment of hypokalemia includes either oral or intravenous potassium replacement and minimizing potassium loss by discontinuing any offending medications. Management of Cushing syndrome involves appropriate surgical resection after localizing the source of excess cortisol production.2 Medical management includes ketoconazole and etomidate, which act by inhibiting multiple steroidogenic enzymes needed for cortisol synthesis. Despite recent advances, the source of ACTH remains occult in up to 20% of ectopic ACTH-secreting Cushing syndrome cases, and serial imaging with high-resolution computed tomography and somatostatin receptor-targeted positron emission tomography-computed tomography is needed to identify the tumor over time.5 Bilateral adrenalectomy may be considered in severe, life-threatening cases.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2025 The Cleveland Clinic Foundation. All Rights Reserved.