ABSTRACT
In September 2024, the American College of Cardiology and American Heart Association updated their 2014 guidelines on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. This comprehensive document reinforces many previous recommendations and provides new evidence and expert opinion that is useful to the perioperative team, including information on risk assessment of ischemic heart disease and associated medical conditions, perioperative medications, cardiac devices, anesthesia, and monitoring. This overview summarizes the major concepts and changes in the new guidelines.
The update reinforces a stepwise approach and proposes a modified algorithm for preoperative risk assessment and management.
Like earlier guidelines, the update suggests preoperative cardiac tests be used judiciously, ie, only when the results will change management.
Prophylactic revascularization is only indicated as it would be in the nonsurgical setting, ie, for acute coronary syndrome and left main coronary artery obstruction greater than 50%.
The guidelines stress a multidisciplinary team–based approach that includes patient preferences.
Very few patients undergoing noncardiac surgery need preoperative interventions such as coronary artery bypass grafting or percutaneous coronary intervention just to get them through surgery unless these procedures are otherwise indicated independent of the need for surgery. This has been an overriding theme of guidelines issued by the American College of Cardiology (ACC), American Heart Association (AHA), and several other professional organizations over the past 3 decades and continued in their latest version, issued in September 2024.1
This article highlights some of the key changes and recommendations of the new guidelines, how they differ from previous and other society guidelines, and ongoing challenges and unresolved issues facing physicians involved in perioperative care.
WHO WROTE THE GUIDELINES?
The original ACC/AHA guidelines for perioperative evaluation and management for non-cardiac surgery were published in 1996 and updated in 2002, 2007, and 2014. In response to advances in preoperative evaluation and perioperative management for noncardiac surgery, the ACC and AHA in conjunction with several other cardiovascular societies updated the guidelines in 2024.1
WHAT ARE THE MAIN RECOMMENDATIONS?
The updated ACC/AHA guidelines1 include the following recommendations:
For preoperative risk assessment
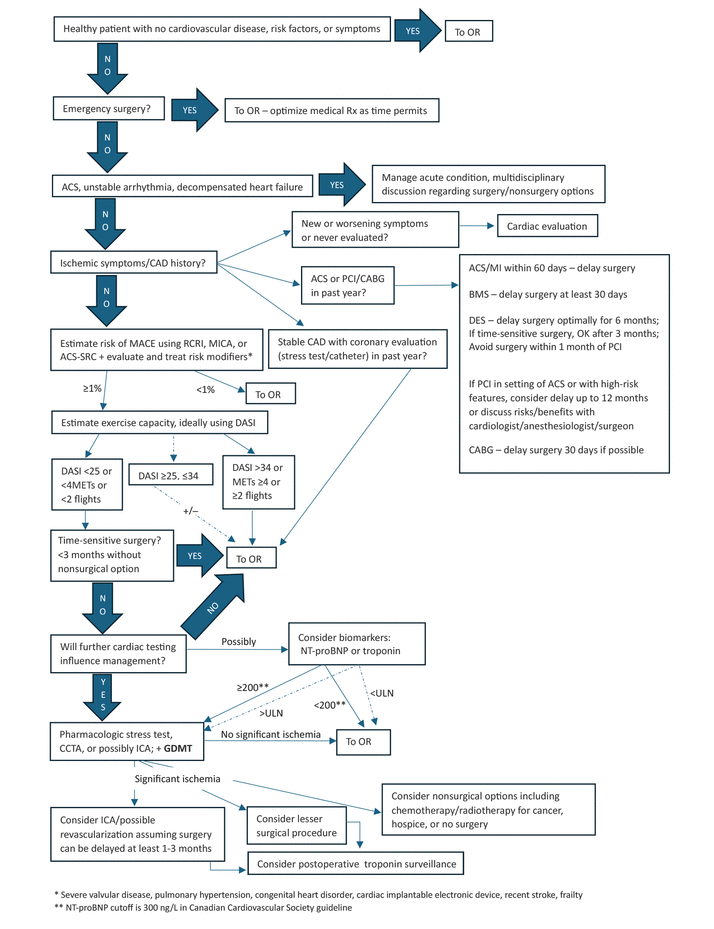
Use a stepwise approach. The new guidelines contain a new algorithm. Figure 1 is a modification of this algorithm that also includes additional items related to a patient with previously undiagnosed or untreated coronary artery disease, prior revascularization, or recent cardiac test results.2
CAD algorithm.
ACS = acute coronary syndrome; ACS-SRC = American College of Surgeons Surgical Risk Calculator; BMS = bare-metal stent; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CCTA = computed tomography angiography; DASI = Duke Activity Status Index; DES = drug-eluting stent; GDMT = guideline-directed medical therapy; ICA = invasive coronary angiography; MACE = major adverse cardiac event; METs = metabolic equivalent of task; MI = myocardial infarction; MICA = Myocardial Infarction or Cardiac Arrest; NT-proBNP = N-terminal brain-type natriuretic peptide; OR = operating room; PCI = percutaneous coronary intervention; RCRI = Revised Cardiac Risk Index; Rx = prescriptions; ULN = upper limit of normal
Reprinted from Cohn SL. Ischemic heart disease. In: Cohn SL, ed. Decision Making in Perioperative Medicine: Clinical Pearls. 2nd ed. McGraw Hill; 2025, with permission of McGraw Hill. Copyright 2025.
Determine timing and urgency of surgery. Definitions for timeframes to go to surgery were changed:
Emergency: Previously meant the patient needed surgery within 6 hours; now it is less than 2 hours
Urgent: Previously 6 to 24 hours; now 2 to 24 hours
Time-sensitive: Previously 1 to 6 weeks; now up to 3 months
Elective: Previously meant surgery could be delayed up to 1 year; now it means indefinitely.
Evaluate and treat unstable cardiac conditions. “Active cardiac conditions”—acute coronary syndrome, decompensated heart failure, and unstable arrhythmias—were included in the 2007 guidelines, removed from the 2014 algorithm (which was for coronary artery disease), but brought back in the 2024 guideline. Severe valvular heart disease was included in a new category of risk modifiers (see below). If the patient has one of these conditions, elective surgery should be postponed pending evaluation and treatment for these conditions.
Estimate risk of major adverse cardiac events. The guidelines recommend combining clinical and surgical risk factors and using a calculator to determine whether the patient is at low risk (< 1%) or elevated risk (≥ 1%). The Revised Cardiac Risk Index (RCRI), Myocardial Infarction or Cardiac Arrest (MICA), and American College of Surgeons Surgical Risk Calculator (ACS-SRC) were recommended in 2014, but the 2024 guidelines do not specify any particular calculator. Several others, including the American University of Beirut-HAS2 Risk Index, are listed.
Address risk modifiers. This is a new step, asking about diseases associated with increased risk that need to be evaluated but are not included in most risk calculators:
Severe valvular heart disease
Pulmonary hypertension
Congenital heart disease
Percutaneous coronary intervention or coronary artery bypass grafting
Recent stroke
Cardiac implantable electronic device
Frailty.
Assess functional status and exercise capacity. The algorithm still mentions a cutoff of 4 metabolic equivalents of task, below which a patient is considered to have poor functional capacity. However, how to accurately assess the patient’s activity level has changed.
In the Measurement of Exercise Tolerance Before Surgery (METS) study,3 clinician assessment of self-reported exercise capacity did not correlate with the number of metabolic equivalents achievable on cardiopulmonary exercise testing or predict postoperative complications, whereas a structured questionnaire—the Duke Activity Status Index (DASI)4—did predict complications. A DASI score higher than 34 (of a possible 58.2 points) was associated with low risk.5 Because many patients have scores lower than 34, Fleisher, in a subsequent editorial,6 suggested an option of using 25 points as another cutoff if you are willing to accept a slightly higher complication rate. Using the lower score as a cutoff would potentially avoid many unnecessary stress tests.
In contrast, data from the Basel Perioperative Myocardial Injury (Basel-PMI)7 and the MET-Reevaluation for Perioperative Cardiac Risk (MET-REPAIR)8 trials demonstrated that a patient’s self-reported inability to climb 2 flights of stairs was associated with increased risk of postoperative cardiac complications and death. While this is noted in the text, it is not listed in the algorithm.
Consider using cardiac biomarkers. This is a new step in the algorithm. Natriuretic peptides (brain-type natriuretic peptide [BNP] or N-terminal pro–BNP [NT-proBNP]) or troponin may be considered for patients at elevated risk with poor exercise capacity going for elevated-risk noncardiac surgery to aid in the decision of whether to proceed to further cardiac testing. The ACC/AHA guidelines1 prefer BNP or NT-proBNP, whereas the European Society of Cardiology9 prefers troponin. If these values are normal (BNP < 92 ng/L, NT-proBNP < 300 ng/L as per the Canadian Cardiovascular Society guidelines10 or possibly < 200 ng/L,11 or troponin ≤ the 99th percentile for the assay), the patient is at low risk and no further cardiac testing is indicated. If these levels are elevated, the team needs to discuss whether to proceed with surgery, order further testing, or consider other options.
Order stress tests only if results will change management. This is one of the main themes of the guidelines. Stress testing is not indicated for patients at low risk, those going for low-risk surgery, or those with adequate exercise capacity. The guidelines recommend that stress tests be used judiciously, for the same indications as in the nonsurgical setting. If the results of further testing are significantly abnormal, once again a team discussion is necessary to decide whether to proceed to invasive coronary angiography and possible revascularization or consider a lesser surgical procedure, nonsurgical options, or not proceeding to surgery.
Consider monitoring troponin after surgery in patients at high risk. This is a change from the 2014 guidelines, which said troponin surveillance should be considered only in patients who had signs or symptoms of ischemia. Now if the patient is at high risk, postoperative troponins can be considered.
Prescribe guideline-directed medical therapy. Appropriate medical therapy to optimize the patient’s medical status is emphasized at multiple steps in the algorithm.
Use a multidisciplinary team approach with shared decision-making. The guidelines emphasize the importance of communication and a team approach to decision-making.
For cardiac diseases other than coronary artery disease
Heart failure. Patients with heart failure are at increased risk of postoperative complications. Risk is higher still in those with heart failure with reduced ejection fraction and those with symptoms. Transthoracic echocardiography is indicated for new dyspnea or worsening symptoms, but not to routinely evaluate left ventricular function in patients without symptoms whose condition is clinically stable. Guideline-directed medical therapy should be continued perioperatively unless contraindicated. An exception is sodium-glucose cotransporter inhibitors, which should be withheld for 3 to 4 days before surgery in patients with heart failure to reduce the risk of perioperative metabolic acidosis.
Valvular heart disease. Transthoracic echocardiography is indicated for patients with known or suspected moderate-to-severe valvular heart disease. If a patient has no symptoms, their condition is clinically stable, and they have had an echocardiogram in the past year, it is not necessary to repeat it. Patients with severe valvular heart disease, in particular aortic stenosis, should be evaluated for possible valve replacement or repair before elective noncardiac surgery. If no intervention is indicated, they can proceed to surgery with appropriate monitoring and medical therapy.
Pulmonary hypertension and congenital heart disease. Management is complex and beyond the scope of this review. Consult a subspecialist.
Blood pressure. Continue most antihypertensive agents. If blood pressure is 180/110 mm Hg or higher before the day of surgery, consider delaying surgery until it is under better control. The anesthesiologist should maintain an intraoperative mean arterial pressure of at least 60 to 65 mm Hg or systolic blood pressure of at least 90 mm Hg.
Postoperative hypotension (mean arterial pressure < 60 or systolic blood pressure < 90 mm Hg) should be recognized and treated promptly.
Recommendations for perioperative medical management
Beta-blockers. Continue them if the patient is already taking them. If there is a new indication to start a beta-blocker (eg, ischemia on a stress test), start it at least 8 days before surgery to assess tolerability and dose titration, but do not start it on the day of surgery.
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. If the patient has been taking these drugs chronically for hypertension and their blood pressure is controlled, withholding them 24 hours before surgery may limit intraoperative hypotension. If the patient takes them for heart failure with reduced ejection fraction, it is reasonable to continue them perioperatively.
Clonidine. Do not start it before surgery; however, continue it if the patient is already taking it to avoid rebound hypertension.
Statins. Continue statins in patients already taking them. If the patient is not already taking them, start them prophylactically for patients with an indication for them such as hyperlipidemia, diabetes mellitus, peripheral artery disease, or high 10-year cardiovascular risk.
Sodium-glucose cotransporter inhibitors. Withhold them 3 to 4 days (4 days for ertugliflozin) before surgery to minimize risk of euglycemic ketoacidosis.
Timing of noncardiac surgery after percutaneous coronary intervention
After balloon angioplasty without a stent, delay noncardiac surgery for at least 14 days.
After drug-eluting stent placement for acute coronary syndrome, elective noncardiac surgery should be delayed for at least 12 months. A 12-month delay between percutaneous coronary intervention and non-cardiac surgery may also be appropriate for patients undergoing complex drug-eluting stent placement (eg, bifurcation stents, long stent lengths, multivessel percutaneous coronary intervention). The European Society of Cardiology guidelines9 say that patients have a high risk of perioperative stent thrombosis if they have any of the following: history of recurrent myocardial infarction, history of stent thrombosis under antiplatelet therapy, reduced left ventricular ejection fraction (< 40%), poorly controlled diabetes, severely impaired renal function or on hemodialysis, recent complex percutaneous coronary intervention (ie, severely calcified lesion, left main percutaneous coronary intervention, chronic total occlusion, bifurcational [crush] technique, bypass graft percutaneous coronary intervention), stent malapposition, or residual dissection.
After drug-eluting stent placement for chronic coronary disease, it is reasonable to delay elective noncardiac surgery for at least 6 months.
After drug-eluting stent placement, time-sensitive noncardiac surgery may be considered at least 3 months after percutaneous coronary intervention if the risk of delaying surgery outweighs the risk of perioperative major adverse cardiac events, and as early as 1 month after stent placement for chronic coronary disease in the European Society of Cardiology guidelines.9
After recent (≤ 30 days) placement of a bare-metal or drug-eluting stent, elective noncardiac surgery requiring interruption of 1 or more antiplatelet agents is harmful, posing a risk of stent thrombosis and ischemic complications.
Perioperative antiplatelet management after percutaneous coronary intervention
Continue aspirin perioperatively, if possible, considering bleeding risk and thrombotic risk, in all patients who have stents regardless of stent type or time of placement.
Continue dual antiplatelet therapy when possible if noncardiac surgery is required within 30 days of bare-metal stent or 3 months of drug-eluting stent placement.
In patients with prior percutaneous coronary intervention on oral anticoagulant monotherapy that must be stopped preoperatively, consider substituting aspirin until the oral anticoagulant can be resumed.
If antiplatelet drugs need to be stopped before surgery, stop prasugrel 7 days before, clopidogrel 5 days before, ticagrelor 3 days before, and aspirin 4 to 5 days before. Ticagrelor is the only reversible platelet inhibitor in this group.
Perioperative antiplatelet management with no prior percutaneous coronary intervention
If the patient is taking aspirin for secondary prevention (because of chronic coronary disease, prior myocardial infarction, or cerebrovascular accident), it may be reasonable to continue it if the risk of cardiac events outweighs the risk of bleeding.
If the patient is taking aspirin for primary prevention and not undergoing carotid surgery, it should be discontinued. If not on aspirin in these settings, initiation of aspirin preoperatively is not indicated.
Bridging anticoagulation
Patients on warfarin. In patients with cardiovascular disease and high thrombotic risk who need to interrupt their vitamin K antagonist (eg, warfarin), preoperative bridging with parenteral heparin (usually low-molecular-weight heparin) can be used. Periprocedural bridging is not indicated in most patients due to increased risk of bleeding.
Patients on direct-acting oral anticoagulants. Patients who need to stop a direct-acting oral anticoagulant preoperatively do not require bridging because these drugs have shorter half-lives than warfarin, and therefore the patient will be without full anticoagulation for a shorter time.
Other recommendations
The guidelines provide recommendations for preoperative management of blood glucose, anemia, sleep apnea, stroke, type of anesthesia, tranexamic acid, cardiac implantable electronic devices, pulmonary artery catheters, transesophageal echocardiography, and body temperature, as well as pain management, postoperative atrial fibrillation, and management of myocardial injury after noncardiac surgery, but it is beyond the scope of this review to discuss them.
WHAT IS DIFFERENT FROM PRIOR GUIDELINES?
The updated guidelines1 differ from previous versions on the following points:
Differences in definitions of urgency and timing of surgery
Use of a structured questionnaire or stair climbing to assess exercise capacity
Inclusion of risk modifiers
Use of biomarkers to aid in decision-making for further cardiac tests
Consideration of coronary computed tomography angiography as an alternative to pharmacologic stress testing
Specific recommendations for revascularization before surgery, ie, acute coronary syndrome and greater than 50% occlusion of the left main coronary artery
Consideration of postoperative troponin surveillance in patients at high risk
Medication management recommendations.
DO OTHER SOCIETIES AGREE OR DISAGREE?
The 2022 European Society of Cardiology guidelines9 are similar in many ways to the ACC/AHA ones,1 but they are more liberal in their recommendations for transthoracic echocardiography and stress tests. They also prefer preoperative troponin measurements rather than natriuretic peptides, whereas the ACC/AHA guidelines prefer natriuretic peptides. They also note that after percutaneous coronary intervention, a patient can proceed to time-sensitive surgery after just 1 month of dual antiplatelet therapy, as opposed to 3 months in the ACC/AHA guidelines.
The 2017 Canadian Cardiovascular Society guidelines10 are very different from the ACC/AHA and European Society of Cardiology guidelines in that they specify using the Revised Cardiac Risk Index as the only validated calculator, recommend using preoperative BNP and NT-proBNP measurements as well as postoperative troponin and electrocardiograms for patients at increased risk, not recommending any cardiac tests (transthoracic echocardiography or stress tests), and withholding angiotensin-converting enzyme inhibitor and angiotensin receptor blocker medications at least 24 hours before surgery for all patients.
WHEN WOULD THE GUIDELINES NOT APPLY?
The 2024 ACC/AHA guidelines provide a framework to assist clinicians with cardiac evaluation and management for patients undergoing noncardiac surgery. However, they cannot address all clinical scenarios and nuances. For example, urgency of the surgery may force the clinician to deviate from standard treatment protocols, and high bleeding risks may result in stopping antiplatelet therapy despite recommendations to continue it. Decisions need to be individualized, with the guidelines serving as the main clinical support tool.
Additionally, many of the ACC/AHA recommendations are class 2, meaning that something may be considered or may be reasonable, giving the clinician the option to do something or not. Clinical experience and discussion with the surgical team and patient play a role in these decisions.
WHAT IS THE EXPECTED CLINICAL IMPACT?
The updated ACC/AHA guidelines provide a streamlined approach to the management of the patient undergoing noncardiac surgery. For those clinicians following the perioperative literature who have already incorporated data from various clinical trials since 2014, the new guidelines will most likely reinforce their practice. However, for those who were using the 2014 guidelines and unaware of newer studies, the updated algorithm represents a major change in terms of evaluating exercise capacity, using preoperative biomarkers, and considering postoperative troponin surveillance. They also update the timing of surgery after percutaneous coronary intervention and management of antiplatelet therapy.
HOW WILL THIS CHANGE DAILY PRACTICE?
Following a stepwise approach, using a more structured questionnaire, and incorporating cardiac biomarkers in decision-making will hopefully lead to better risk assessment and minimize unnecessary stress tests. Appropriate medication management, careful attention to and treatment of hypotension, and vigilant postoperative monitoring and follow-up may improve outcomes. Incorporation of artificial intelligence and machine-learning may improve risk assessment, but future studies are needed to evaluate risk-reduction strategies.
DISCLOSURES
Dr. Cohn reports no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2025 The Cleveland Clinic Foundation. All Rights Reserved.

Clicking the link below will connect you to begin the credit-claiming process for CME and MOC. After clicking on the link, scroll to the bottom of the page and click on “Complete the CME/MOC Process.” You will need your myCME login information to access this.