ABSTRACT
Whether subclinical hypothyroidism should be treated in elderly patients (≥ 65 years) is controversial. The authors argue for a personalized, wait-and-see approach rather than universal treatment, pointing out that randomized clinical trials have not shown that levothyroxine treatment makes any difference in terms of hard clinical end points, quality of life, or hypothyroid symptom relief in elderly patients with this condition.
Subclinical hypothyroidism is categorized as either mild (grade 1; thyroid-stimulating hormone [TSH] level 4.0–10.0 mIU/L) or severe (grade 2; TSH > 10 mIU/L).
High TSH levels in patients older than 65 years may be due to aging and do not necessarily require treatment.
The French Endocrine Society proposes using the patient’s age divided by 10 as the upper limit of normal for TSH (in mIU/L) when screening and following elderly patients.
Studies have not found levothyroxine replacement therapy to make any significant clinical difference in most cases of mild subclinical hypothyroidism in older patients. However, lack of consistent age-appropriate TSH screening methods limits definitive conclusions.
In most mild cases, TSH can be remeasured 2 to 3 months after diagnosis. Treatment decisions are individualized, and potential risks and benefits must be considered.
Most patients with subclinical hypothyroidism do not need to be treated for it, and for many, it may be a normal part of aging and can be monitored without active intervention.
The US Preventive Services Task Force defines subclinical hypothyroidism as an elevated serum thyrotropin (thyroid-stimulating hormone, TSH) level (> 4.50 mIU/L), but with a normal free thyroxine (T4) level.1 Despite the term subclinical, symptoms may or may not be present, although they tend to be mild and nonspecific.
Guidelines for diagnosing and managing overt hypothyroidism (in which the TSH level is elevated and the T4 level is low) enjoy broad consensus.2,3 However, whether to treat subclinical hypothyroidism is controversial, especially in people 65 and older. A host of factors, including age, can affect TSH levels. Adding to the challenge, the ideal TSH cutoff point for initiating treatment remains a topic of debate.4 Mildly elevated TSH does not necessarily lead to long-term adverse consequences, and overtreatment can increase the risks of fractures, cardiovascular disease, and dysrhythmias.5
Here, we review the challenges of managing subclinical hypothyroidism in the elderly (age 65 and older) and argue against routinely treating it with levothyroxine in this age group. We do not cover how to manage it in younger adults, women of childbearing age, or children.
PRIMARILY A LABORATORY DIAGNOSIS
The clinical presentation of hypothyroidism often deviates from the classic textbook description. Carlé et al6 report that tiredness is the most important symptom of overt hypothyroidism. However, many patients with subclinical hypothyroidism experience no symptoms or only nonspecific symptoms. Consequently, subclinical hypothyroidism is primarily a biochemical or laboratory diagnosis.
TSH is more sensitive than T4
TSH levels have a log-linear inverse relationship with T4 and triiodothyronine (T3) levels, so that a 2-fold decrease in T4 results in a 100-fold increase in TSH.7 Therefore, TSH is more sensitive to changes in thyroid function.
Free T4 measurements, complementing TSH, help classify hypothyroidism as overt or subclinical and determine the need for treatment. Free T4 is a better marker of hormone action than total T4, as the latter is 99.97% bound to proteins (thyroid-binding globulin, transthyretin, prealbumin, and albumin), and conditions that alter these binding proteins affect the accuracy of total T4 measurements.8 Also, unbound (free) T4 is the biologically active fraction and is widely accepted as a better activity marker.2
T3 is 99.7% protein-bound, and the free T3 level (the other 0.3%) is theorized to be a better marker than total T3. However, free T3 has a lower concentration than free T4 and a weaker affinity for protein carriers, which renders it more susceptible to free fatty acids (which inhibit its binding to its receptor) and drug interactions.9 Consequently, the precision and reproducibility of free T3 measurements are less ideal than those of free T4. Also, T3 levels tend to be within reference ranges in cases of suspected hypothyroidism or elevated TSH and so have limited clinical value (Table 1).2,7–9 As a result, in the special cases in which T3 measurements are needed, such as euthyroid sick syndrome, many laboratories prefer total T3 assays rather than free T3.
Advantages and disadvantages of different thyroid function tests
The American2,3 and European thyroid associations10 classify subclinical hypothyroidism as either mild (grade 1) or severe (grade 2) based on the TSH level (Table 2)—about 90% of patients with subclinical hypothyroidism have TSH levels of 10 mIU/L or lower (ie, mild or grade 1).
Classification of subclinical hypothyroidism
Before diagnosing subclinical hypothyroidism, one must make sure the thyroid function has been stable for at least several weeks to exclude transient changes caused by nonthyroidal illness, thyroiditis, or medications.2 We remeasure TSH 2 to 3 months after the first measurement.
COMMON IN THE ELDERLY
Western countries are getting older. Consequently, the prevalence of subclinical hypothyroidism is also rising, reflecting the growing proportion of the population at risk.11
In the third National Health and Nutrition Examination Survey (NHANES III),12 in people age 12 years and older in the United States, the prevalence of subclinical hypothyroidism was 4.3% and the prevalence of overt hypothyroidism, characterized by low free T4 levels, was 0.3%. In the Cardiovascular Health Study,13 in participants age 65 and older, the prevalence of subclinical hypothyroidism was 15%, and it was higher in women than in men.
MANY FACTORS AFFECT TSH LEVELS
When T4 and T3 levels are low, the pituitary gland releases TSH to increase thyroid hormone production, whereas high T4 and T3 levels inhibit TSH release. However, many other factors can affect the TSH level, making abnormal values difficult to interpret (Table 3).14–21 TSH levels are not static but fluctuate within individuals over time,17 owing to a range of factors:
Circadian rhythm. TSH levels peak in the early morning hours and then decline, reaching a trough in the early afternoon and evening
Pulsatile secretion. TSH is secreted in pulses, contributing to further fluctuations
Season. Levels are generally higher in winter and lower in summer
Age. TSH levels mildly increase with age, and elevations may be normal for some individuals12,18
Other factors, which include inconsistencies in testing methods, thyroid antibodies and thyroid hormone resistance,11,19,20 silent or granulomatous thyroiditis, and certain medications.21
Factors that can affect serum thyroid-stimulating hormone (TSH) levels
Genetic differences also affect thyroid hormone levels, so that some patients whose TSH levels are within the normal range can have symptoms while others with slightly elevated TSH might not.22 Also, iodine intake varies in geographic regions.
The complex interplay of factors that influence thyroid hormone regulation makes the distinction between subclinical hypothyroidism and overt hypothyroid disease arbitrary.
IS THE UPPER LIMIT OF NORMAL FOR TSH TOO HIGH?
The true upper limit of normal of TSH to diagnose subclinical hypothyroidism is still debated.14
Currently, the normal reference range for TSH is extrapolated from population-based data, calculated from Gaussian distribution with 95% confidence intervals of studies of people who do not have thyroid disease, thyroid peroxidase antibodies, or thyroglobulin antibodies.23 In most studies, the lower limit of TSH (the 2.5th percentile) was between 0.2 and 0.4 mIU/L, but the upper limit (the 97.5th percentile) varied between 2.4 and 4.2 mIU/L.24
Why was this range so wide? Many people in the studies actually had undiagnosed autoimmune thyroid disease (Hashimoto thyroiditis) or other factors that affect TSH, such as medications, nonthyroidal illness, pregnancy, subacute thyroiditis in the recovery phase, heterophilic antibodies, thyroid hormone resistance, TSH-receptor mutations, bio-inactive TSH, and TSH-producing pituitary tumors, or had their blood drawn at a high point in their circadian rhythm.25 This renders the TSH distribution non-Gaussian, with a tail at the upper end.26 If we take out these confounding factors, and a normal Gaussian distribution with a bell-shaped curve is achieved, the normal reference range becomes 0.4 to 2.5 mIU/L.27
In view of these studies, some have proposed lowering the upper limit of normal from 4.5 to 2.5 mIU/L.24,27 However, Surks et al28 argue against changing the upper limit, estimating that if we lower the upper limit of normal for TSH to 3.0 mIU/L to screen elderly patients with vague symptoms, it will label 22 to 28 million more Americans as having subclinical hypothyroidism without evidence-based therapeutic benefit from this diagnosis. Instead, they suggest measuring the TSH level every 1 to 2 years in patients whose TSH levels are between 2.5 and 4.5 mIU/L.
WHY DOES TSH RISE WITH AGE?
Why TSH increases with age is not known.29 In NHANES III,12 TSH levels increased with age, and, interestingly, so did the prevalence of thyroid peroxidase antibodies and thyroglobulin antibodies. Some 14% of people older than 85 years had TSH levels higher than 4.5 mIU/L.
Hashimoto thyroiditis is the most common condition associated with subclinical hypothyroidism in the elderly, and in almost 90% of cases is characterized by antibodies against thyroid peroxidase and thyroglobulin.11 Patients who had these antibodies progressed to having overt hypothyroidism at the rate of 4.3% per year, compared with only 2.6% in those without these antibodies.30
Once these antibodies are present, however, changes in their levels do not add more information while monitoring subclinical hypothyroidism, due to parallel fluctuation of TSH and thyroid peroxidase antibody.31 These antibodies are not harmful to the thyroid glands; however, the volume of thyroid glands generally shrinks after 50 years of age, and pathology studies have found lymphocytic infiltration of the gland and fibrosis.31 Thus, aging-associated thyroid cellular damage from cellular and humoral immune mechanisms with possible T lymphocytes was suspected.11,32 Changes in iodine absorption and organification have been observed in elderly patients.33 In addition, the normal nocturnal surge in TSH (possibly related to maintenance and repair mechanisms) is partially or completely lost. Thus, nighttime TSH levels are lower but 24-hour TSH levels are higher, to keep the T4 level normal.17 Corticosteroid inhibitory function is also compromised in the elderly and leads to decreased hypothalamic-pituitary-thyroid inhibitory function.11
While a direct correlation between aging and mild thyroid failure is debated, there is evidence to suggest that some aspects of aging may resemble the symptoms and physiologic changes associated with mild hypothyroidism.11 Whether this is a protective mechanism in the elderly or rather represents a diseased hypothalamic-pituitary-thyroid axis is debated.34 In a study in southern Italy, Corsonello et al35 found that older people had lower T4 and T3 levels and higher TSH levels—and so did the children and nieces and nephews of centenarians, suggesting that these changes might be associated with longevity.
Also, elderly patients have less circadian variation in TSH and a weaker response to thyrotropin-releasing hormone compared with the young, changes that are believed to conserve energy, particularly in those with reduced physical activity.11,18
IS SUBCLINICAL HYPOTHYROIDISM HARMFUL? IS TREATMENT BENEFICIAL?
Findings have been inconsistent regarding the potential harms of untreated subclinical hypothyroidism, and evidence that treatment is beneficial is lacking.
Cardiovascular disease
Thyroid hormones play a crucial role in regulating various bodily functions, glucose metabolism, protein synthesis, and cardiovascular function. However, the impact of thyroid hormones on the cardiovascular system, particularly in older adults with subclinical hypothyroidism, is inconsistent.11
In the Rotterdam Study,36 TSH levels higher than 4.0 mIU/L with normal free T4 were associated with higher risks of myocardial infarction (odds ratio 2.3) and aortic calcification (odds ratio 1.7). When antithyroid antibodies were present, patients with subclinical hypothyroidism had greater progression to more severe atherosclerosis than euthyroid individuals.
Japanese men, average age 58.5 years, with TSH higher than 5.0 mIU/L and normal free T4 had a high risk of ischemic heart disease (odds ratio 4.0).37 In Taiwanese people age 20 and older with subclinical hypothyroidism followed for 10 years, the all-cause mortality rate was 30% higher, and the rate of death due to cardiovascular disease was 68% higher than in euthyroid people.38
In contrast, a study that analyzed data from the Cardiovascular Health Study13 found no links between subclinical hypothyroidism and adverse cardiovascular, cerebrovascular, or mortality outcomes. However, the range of TSH values was wide: 4.5 to 20.0 mIU/L.
A meta-analysis39 found significantly greater risks for coronary heart disease morbidity and mortality in those with TSH levels 10 mIU/L and higher, and the risks increased significantly in those age 65 to 79 years, but not at age 80 or above, compared with euthyroid participants. In other meta-analyses that used data from the Thyroid Studies Collaboration cohort,40–42 there was no association between subclinical hypothyroidism (with TSH levels in the range of 4.5–19.9 mIU/L) and stroke, atrial fibrillation, or heart failure.19 Overall, the mortality rate was not higher than in euthyroid patients. However, the subgroup with TSH levels 10 mIU/L or higher had higher risks of heart failure, coronary heart disease events, and death from coronary heart disease than the euthyroid group. In addition, the risks of death from stroke and coronary heart disease were higher in those with TSH levels of 7.0 to 9.9 mIU/L.39,40
In a post hoc analysis of the Prospective Study of Pravastatin in the Elderly at Risk,44 patients with subclinical hypothyroidism (age range 70–82 years) had significantly higher rates of heart failure and death, but only those with TSH levels higher than 10 mIU/L.
Razvi et al45 performed a meta-analysis and found no difference in ischemic heart disease morbidity or mortality in patients age 65 or older with subclinical hypothyroidism, but did find increased mortality in patients younger than 65 years.
Cognitive dysfunction and depression
Thyroid hormones are essential for brain development; however, whether subclinical hypothyroidism affects cognition in elderly patients is questioned, and studies of this matter are inconclusive.11
Pasqualetti et al46 conducted a meta-analysis of prospective and cross-sectional studies and found that subclinical hypothyroidism was associated with cognitive impairment in patients younger than 75, but not older. However, in the 2015 US Preventive Services Task Force study, Rugge et al1 reviewed trials of treatment vs placebo for subclinical hypothyroidism (TSH 4.5–10 mIU/L or ≥ 10 mIU/L) and found no effects on cognitive skills or performance, speed or capacity of language processing, or psychomotor tests of executive functions. They rated the overall quality of evidence as “poor.”
Dyslipidemia
Rugge et al1 found only small changes in the average total cholesterol and low-density lipoprotein cholesterol levels with treatment, and no significant differences in high-density lipoprotein cholesterol levels or triglyceride levels between the treated and untreated groups.
Blood pressure
Rugge et al1 found no evidence that treating subclinical hypothyroidism affected blood pressure in patients with TSH levels higher than 3.6 mIU/L.
Mortality
Some studies13 found no significant association between subclinical hypothyroidism and mortality.
Quality of life and symptoms
The only large study that delved into hypothyroid symptoms such as dry skin, poor memory, cognitive slowing, muscle weakness, cold feelings, voice changes, constipation, and puffy eyes was the cross-sectional Colorado Thyroid Disease Prevalence Study.47 Interestingly, 25% of individuals with overt hypothyroidism, 20% of those with subclinical hypothyroidism, and 17% of euthyroid individuals reported 4 or more symptoms commonly associated with hypothyroidism.
The Thyroid Hormone Replacement for Untreated Older Adults With Subclinical Hypothyroidism (TRUST) trial4 included 737 adults 65 or older with TSH levels ranging from 4.6 to 19.99 mIU/L and normal free T4 levels, who were given levothyroxine (median dose 50 μg) or placebo. At 12 months, in the treated group, TSH levels had declined from 6.40 mIU/L to 3.63 mIU/L, but there was no clinical difference in the mean changes of hypothyroid symptoms score or in the tiredness score. These findings raise the question of whether subclinical hypothyroidism is a true disease or just a biochemical cutoff.
Kidney function
In theory, thyroid deficiency could affect kidney function by lowering cardiac output, although evidence does not support an association between subclinical hypothyroidism and kidney dysfunction.48 However, patients on hemodialysis who had subclinical hypothyroidism had a higher mortality rate.49
Neuromuscular effects
Although there have been suggestions that neuromuscular symptoms are common in patients with subclinical hypothyroidism, more definitive answers are needed for those with TSH 10 mIU/L or below.50 In several studies in elderly patients, there was no association between subclinical hypothyroidism and low bone mineral density, fracture risk, or frailty compared with euthyroid patients (reviewed by Biondi et al19).
HARMS OF OVERTREATMENT
Many patients with hypothyroidism are being overtreated. Biondi and Cooper26 estimate that the overtreatment rate of subclinical hypothyroidism exceeds 20%. This overtreatment can lead to low TSH levels, potentially causing thyrotoxicosis in elderly patients. In 2 studies, 22% to 23% of patients on levothyroxine replacement had suppressed TSH levels.47,51 At the Massachusetts General Hospital Thyroid Clinic, 14% of patients on levothyroxine therapy had suppressed TSH levels.52
Subclinical or overt thyrotoxicosis can lead to atrial fibrillation, cardiovascular events, reduced bone density, fractures, and increased mortality. A study in Denmark53 followed a cohort of patients with overt and subclinical hypothyroidism every 6 months for a median of 7.2 years and found that the mortality rates of those who were overtreated with levothyroxine so that their TSH levels were lower than 0.3 mIU/L increased with duration of overtreatment.
GUIDELINES AND RECOMMENDATIONS
Several medical organizations have published guidelines for diagnosing and managing subclinical hypothyroidism. These differ somewhat, as medical research evolves and there is as of yet no universal consensus.
The European Thyroid Association, in its 2013 guideline,10 recommends classifying subclinical hypothyroidism as mild or severe according to TSH level; if mild, it suggests no treatment but repeating this measurement 2 to 3 months later, along with thyroperoxidase antibodies. If the patient is younger (< 65–70) and has TSH higher than 10 mIU/L, treatment should be started. If a patient in this younger group has TSH lower than 10 mIU/L and normal free T4 but has symptoms, a trial of thyroxine can be considered. If the TSH level is normal after 3 to 4 months of thyroid replacement therapy, then symptoms should be reevaluated and therapy should be stopped if no symptomatic change is observed.
In older people, the European Thyroid Association recommends using age-specific local reference ranges for TSH to diagnose subclinical hypothyroidism (eg, TSH 4–7 mIU/L for those older than 80 years).10 In patients older than 80 to 85 years with TSH 10 mIU/L or less, a wait-and-see strategy should be prioritized before starting replacement therapy, as deleterious effects of subclinical hypothyroidism on cardiovascular risk vanish in this group. If treatment is to be started in older patients (> 65–70 years), the target TSH range should be the lower half of the reference range, or 0.4 to 2.5 mIU/L. For patients without cardiac disease, a weight-based dose of 1.5 μg/kg/day should be used. In patients with preexisting cardiac disease, they say to start levothyroxine treatment low at 25 to 50 μg/day and increase it every 2 to 3 weeks to slowly bring TSH down to target.
In patients with persistent subclinical hypothyroidism, thyroid function tests should be checked every 6 months for the first 2 years, and then annually.
The American Thyroid Association and American Association of Clinical Endocrinology 2012 guideline2 and their 2014 update3 echo the European classification of mild vs severe subclinical hypothyroidism. In severe subclinical hypothyroidism, thyroxine treatment is generally supported, similar to the European recommendation. However, the American guideline is less specific regarding mild subclinical hypothyroidism. It recommends considering individual factors such as symptoms, thyroid peroxidase antibody status, and evidence of cardiovascular disease, especially in patients younger than 65 years.
The 2014 American Thyroid Association update3 recommends starting low and titrating slowly in elderly patients with hypothyroidism, and recommends raising the TSH target range to 4 to 6 mIU/L in persons 70 to 80 years old. It also cautions against iatrogenic thyrotoxicosis from overtreatment with levothyroxine, especially avoiding TSH levels lower than 0.1 mIU/L in older and postmenopausal women to avoid cardiac and bone deleterious effects.
Other organizations22,54–56 have also issued recommendations regarding hypothyroidism diagnosis and treatment. Although these recommendations are not specific to subclinical hypothyroidism in the elderly, they generally concur with those above (Table 4).1–3,10,54,55
Guidelines and recommendations on managing subclinical hypothyroidism
MANAGEMENT CHALLENGES
There are no uniform national guidelines on screening elderly patients with blood TSH levels for thyroid disease.
Although the association between increasing TSH and aging is well described,2,10,47 the practice of using age-adjusted TSH reference ranges to diagnose elderly patients with thyroid diseases is not currently routine.57 As a result, the current TSH normal range (0.4–4.5 mIU/L) is inappropriately used to diagnose subclinical hypothyroidism in patients older than 65 years, and this overestimates the prevalence of subclinical hypothyroidism and also leads to overtreatment.
Moreover, Danese et al58 estimated the cost of routine screening in women older than 35 years every 5 years at approximately $9,200 per quality-adjusted year of life (this was in 1996, and the cost would be higher now), raising concerns about the feasibility and potential drawbacks of universal screening.
The concept of using a TSH cutoff value can also be deceiving. The test is based on a sample of healthy volunteers without thyroid disease or thyroid antibodies, representative of a larger population and calculated with statistical methodology. However, many factors can affect this arbitrary reference range (Table 3).17,59 For example, studies in urban populations over age 55 without thyroid peroxidase antibodies or other factors show higher TSH levels in White and Mexican American individuals than in Black individuals.12,21 Scobbo et al60 demonstrated that TSH fluctuates throughout the day, being significantly higher before breakfast (7:30–9:00 am) than after breakfast (10:30 am–12:00 pm), with an average decline of 26.4%.
Another problem with the use of a TSH cutoff to define subclinical hypothyroidism is that TSH changes can be transient and reversible. In a large retrospective longitudinal study,61 57.9% of patients with subclinical hypothyroidism reverted to euthyroid TSH levels during a median follow-up of 36 months. Unfortunately, most of the studies dealing with risks and benefits associated with subclinical hypothyroidism included patients who had no repeat TSH assessments to confirm the persistence of TSH elevation.21,29 This raises the question of inconsistency in the benefits and risks of treating subclinical hypothyroidism. On top of this, observational studies did not address TSH status while receiving treatment.13 All of these uncertainties contribute to the debate about the clinical relevance of subclinical hypothyroidism, especially in grade 1 subclinical hypothyroidism, and in older patients.
In summary, factors such as the time of day, ethnicity, and inconsistent TSH levels complicate accurate subclinical hypothyroidism diagnosis. Varied study methodologies and a lack of longitudinal TSH data further obfuscate the issue. The absence of age-appropriate diagnostic guidelines and consensus makes establishing clear evidence-based benefits difficult.
PHYSICIAN DECISION-MAKING AND ALTERNATIVES
While some studies have linked untreated subclinical hypothyroidism to cardiovascular issues and heart failure, the US Preventive Services Task Force reviewed multiple studies and found no significant clinical benefit from treatment in grade 1 subclinical hypothyroidism.1 The 2017 TRUST randomized clinical trial supported these findings.4
To personalize the diagnosis and treatment, the French Endocrine Society56 proposed a novel approach in their 2019 consensus statement—using the formula patient age divided by 10 to establish the upper limit of normal for TSH in patients older than 60. This approach acknowledges the potential for natural changes in TSH levels with age. Consequently, for an 80-year-old, the upper limit for TSH would be 8 mIU/L, which differs from the standard cutoff used for younger populations.
Recently, Kuś et al16 reported a novel approach to personalizing TSH intervals: using a polygenic score system based on 59 genetic variants to calculate TSH reference ranges. This predicted 9.2% to 11.1% of total variance in TSH concentrations, compared with 2.4% to 2.7% with free T4. This reflects the idea that different individuals have different set points in their hypothalamic-pituitary-thyroid axis, and thus can have higher TSH concentrations at the same free T4 levels.59
In older patients, Calsolaro et al11 recommend treating severe subclinical hypothyroidism with thyroxine. However, in patients with mild subclinical hypothyroidism, starting levothyroxine can be considered on a case-by-case basis according to symptoms, frailty, cardiovascular risk factors, and personal preferences. If a patient needs treatment, they recommend following the professional guidelines. Alternatively, mild subclinical hypothyroidism can be monitored without therapy by repeating laboratory tests every 3 to 6 months to monitor progression of overt disease and treated if TSH rises to more than 10 mIU/L.2,10,58
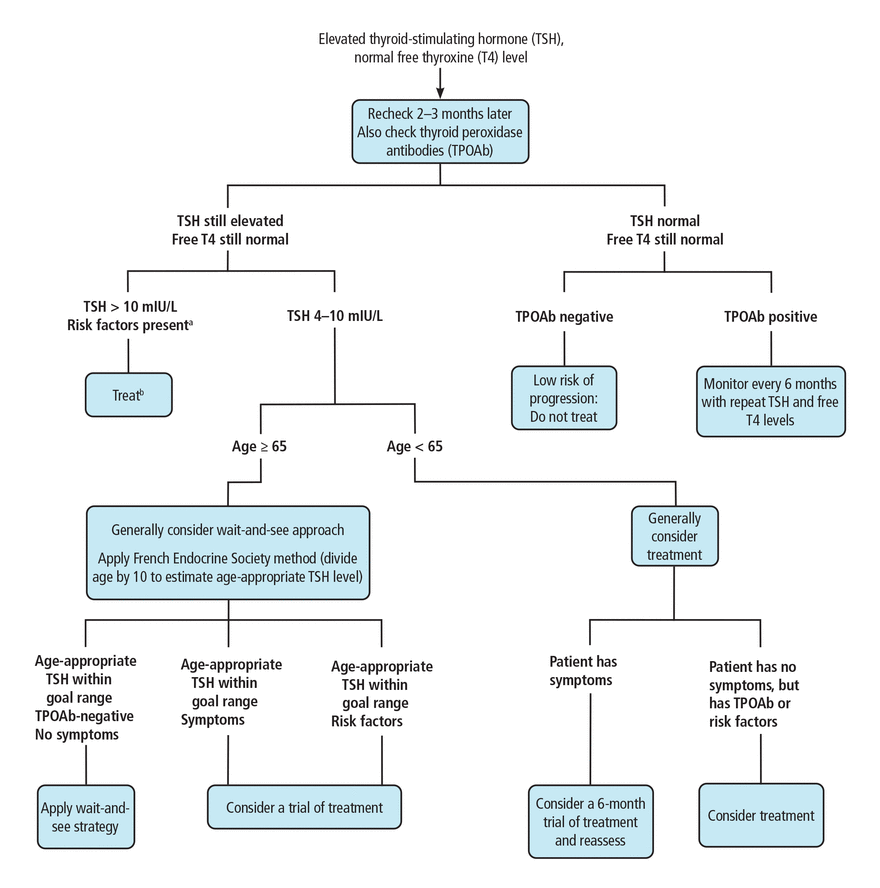
We propose an approach that combines the professional society guidelines and the French Endocrine Society 2019 consensus statement56 with an age-based TSH cutoff (Figure 1). When the decision becomes complicated, consultation with a team including an endocrinologist and geriatrician is recommended.
Flow chart for clinical decision-making in subclinical hypothyroidism.
aRisk factors: TPOAb-positive, goiter, atherosclerotic cardiovascular disease, heart failure, or associated risk factors for these diseases.
bOral levothyroxine daily is the treatment of choice. For patients with cardiac disease, 1.5 μg/kg/day should be used. For elderly patients with cardiac disease, a dose of 25–50 μg/day is recommended. Increase dose by 12.5 to 25 μg/day every 2 to 3 weeks. Target TSH range is 0.4 to 2.5 mIU/L.
A PERSONALIZED APPROACH
Subclinical hypothyroidism is common in the elderly and is going to be more so in the coming decades. Its management requires a personalized approach that weights the potential benefits and risks, as current evidence does not show a clear difference in outcomes if mild subclinical hypothyroidism is treated or not. Age-adjusted TSH reference ranges, consideration of individual circumstances, and a wait-and-see approach for mild cases might be more suitable than a universal treatment strategy.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Acknowledgments
The authors thank their colleagues in the divisions of Geriatrics and Endocrinology for their insights and recommendations on this topic and their efforts in reviewing and strengthening the content of this manuscript.
- Copyright © 2025 The Cleveland Clinic Foundation. All Rights Reserved.