ABSTRACT
Pulmonary embolism (PE) has a significant impact on right-sided heart function. Clinical presentation can range from no involvement of the right ventricle to right ventricular dysfunction, cardiogenic shock, and even cardiac arrest. The authors explore the pathophysiology of PE-induced right ventricular failure, emphasizing the mechanisms by which PE contributes to dysfunction, current diagnostic tools for risk stratification, and the importance of timely diagnosis. The primary focus is on strategies for managing right ventricular failure secondary to PE, including medical, percutaneous interventional, and surgical options. Recent advances in the field are also noted, including emerging therapies and evolving treatment algorithms.
Inpatient mortality for patients with high-risk PE is as high as 42.1% and is primarily due to right ventricular dysfunction from a sudden rise in right ventricular afterload.
Risk of mortality is classified as low, intermediate (intermediate low-risk and intermediate high-risk), and high.
Management of PE with right ventricular involvement of varying severity requires prompt and concomitant integration of several approaches: management of hemodynamics (preload and afterload), reperfusion, pharmacologic support, supportive care, and, in refractory cases, use of mechanical circulatory support and advanced therapy.
Pulmonary embolism (PE) significantly impacts right-sided heart function. Clinical presentation can range from no involvement of the right ventricle to right ventricular dysfunction, cardiogenic shock, and even cardiac arrest. In a retrospective subset from the Pulmonary Embolism Response Team Consortium Registry,1 inpatient mortality was as high as 42.1% in patients with high-risk PE with hemodynamic collapse (termed catastrophic PE). Management requires critical evaluation, risk stratification, and multidisciplinary care that can include medical, percutaneous interventional, and surgical options. This article predominantly discusses patients classified as having intermediate- and high-risk PE, given the associated hemodynamics and involvement of the right ventricle.
RIGHT VENTRICULAR FAILURE IN ACUTE PE
Acute PE is associated with an elevated risk of death, primarily due to acute right ventricular dysfunction resulting from a sudden rise in right ventricular afterload.2 Vascular obstruction caused by thrombus in the pulmonary arterial circulation can lead to significant increases in pulmonary pressures and pulmonary vascular resistance, raising right ventricular afterload. In PE, hypoxemia and pulmonary vasoconstrictors also contribute to pulmonary vascular resistance. The crescent-shaped right ventricle, characterized by fine layers of myofibrils arranged in series for volume expansion and enhanced compliance compared with the left ventricle, is designed to tolerate preload changes and is compliant in low-pressure systems. It struggles to adapt rapidly to acute elevations in circulatory pressures and has limited capacity to adapt to sudden increases in afterload.
During an acute PE, the right ventricle attempts to preserve right ventricle–pulmonary artery coupling and maintain stroke volume and cardiac output by dilating and altering its geometry.3 However, the right ventricle cannot endure consistently elevated pressures, which leads to further right ventricular dilation and acute septal deviation toward the left ventricle, giving rise to altered ventricular interdependence—a phenomenon where the performance of one ventricle is influenced by the other due to the shared interventricular septum. Consequently, the right ventricle exerts pressure on the interventricular septum, impairing filling of the left ventricle, decreasing left ventricular preload, and reducing blood supply to the coronary arteries.
The increase in right ventricular intramural pressure and wall tension also causes straightening of the wall of the right ventricle, leading to decreased right coronary artery perfusion and right ventricular ischemia, even in individuals without preexisting coronary disease.2 Furthermore, with persistent right ventricular dilation and enlargement, the tricuspid valve annulus may expand, resulting in inadequate closure of the valve leaflets and subsequent secondary tricuspid regurgitation. These effects create a cycle of ischemia, compounded by decreased oxygenation from obstructive thrombotic material, resulting in a drop in blood pressure and hemodynamic changes, all of which may manifest as syncope, hypotension, cardiogenic shock, and cardiac arrest (Figure 1).2,4 Moreover, this dysfunction permits retrograde blood flow into the right atrium, compromising right ventricular filling, elevating right atrial pressure, and contributing to progressive venous congestion.
DIAGNOSTIC AND PROGNOSTIC TOOLS IN ACUTE PE
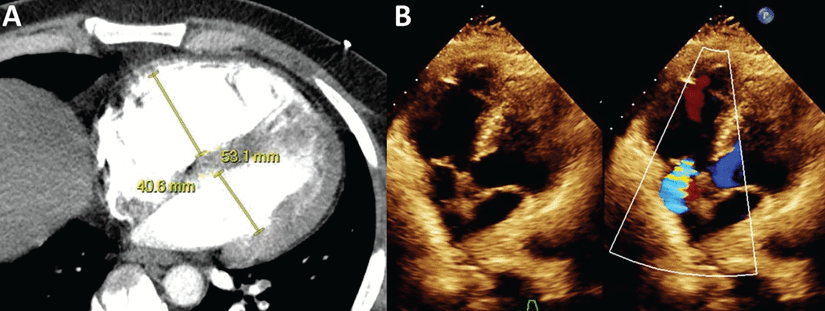
Signs of right ventricular involvement can be observed through laboratory and imaging studies. Computed tomography pulmonary angiography has been a cornerstone in the diagnosis of acute PE due to its detailed contrast enhancement of pulmonary vasculature. It is also a valuable tool in assessment of the right ventricle. Per the 2019 European Respiratory Society (ERS) guidelines, an increase in the right ventricle-to-left ventricle diameter ratio greater than 1 found with computed tomography pulmonary angiography (Figure 2) is indicative of acute right ventricular dysfunction, and is associated with increased risk of adverse outcomes and all-cause and PE-related mortality.5–7 Additional findings that may be supportive of acute right ventricular failure in certain clinical settings include septal straightening or bowing, reflux of contrast in the inferior caval vein, and hepatojugular reflux of contrast into the inferior caval vein. Thrombus load and central location have not shown a consistent association with all-cause mortality.6
(A) Computed tomography and (B) echocardiogram showing an increased right ventricle–to–left ventricle ratio, which is used to assess right ventricular strain and dysfunction for risk stratification of pulmonary embolism.
Echocardiography is invaluable in the assessment of right ventricular dysfunction in acute PE as it can identify numerous findings suggestive of right ventricular dysfunction5,7:
Enlarged right ventricle
Dilated right ventricle with right ventricle-to-left ventricle ratio (> 1.0)
McConnell sign (right ventricular free wall akinesis with the apex spared)
Flattened intraventricular septum
Distended inferior caval vein with decreased inspiratory collapsibility
Decreased tricuspid annular plane systolic excursion (< 16 mm)
Decreased peak systolic velocity of tricuspid annulus
Right heart mobile thrombus or clot in transit
60/60 sign (coexistence of acceleration time of pulmonary ejection less than 60 ms and midsystolic notch with mildly elevated [< 60 mm Hg] peak systolic gradient at the tricuspid valve).
Low left ventricular outflow tract velocity time integral of 15 cm or less and right ventricular outflow tract velocity time integral less than 9.5 cm have also been associated with adverse outcomes.8,9
Biomarkers that have been proposed as tools for diagnosis and prognosis of patients with acute PE include troponins (marker of myocardial injury) and natriuretic peptides (marker of right ventricular dysfunction).7
TRIAGING AND RISK STRATIFICATION
Initial management of patients with PE begins with triaging and risk stratification using clinical assessment tools including the Pulmonary Embolism Severity Index (PESI) score or its simplified version (sPESI), cardiac biomarkers, and imaging (Figure 3).1,7
Algorithm of our initial assessment and risk stratification of pulmonary embolism based on Pulmonary Embolism Severity Index (PESI) score, cardiac markers (troponin or brain natriuretic peptide), right ventricular strain on imaging (transthoracic echocardiography or computed tomography pulmonary angiography), and hemodynamic instability (cardiac arrest, obstructive shock, or persistent hypotension). Note the addition of a subcategory of high-risk pulmonary embolism, termed catastrophic pulmonary embolism (those with hemodynamic collapse).
Severity of PE and risk of early death are stratified as high, intermediate (intermediate low-risk and intermediate high-risk), and low. Patients classified with high-risk PE are hemodynamically unstable, as defined by cardiac arrest, shock, or hypotension. Patients classified with intermediate-risk PE have signs of right ventricular involvement, while patients classified as low risk do not. Patients with intermediate high-risk PE are hemodynamically stable but have abnormalities in all 3 indicators of risk—clinical (PESI III–V or sPESI ≥ I), laboratory (elevated troponin), and imaging parameters indicative of right ventricular dysfunction (shown on computed tomography pulmonary angiography or echocardiography). Patients classified with intermediate low-risk PE have at least clinically severe presentation and 1 or none of the other parameters.7 In the registry study noted earlier, the term catastrophic PE was introduced as a further classification of patients with high-risk PE and hemodynamic collapse, particularly those requiring vasopressors or experiencing cardiac arrest.1
ESSENTIALS OF MANAGEMENT
Management of PE with right ventricular involvement of varying severity requires prompt, concomitant integration of clinical care focused on hemodynamics (preload and afterload), reperfusion, pharmacologic support, supportive care, and, in refractory cases, use of mechanical circulatory support and advanced therapy (Table 1).7,10
Principles of management options of right ventricular failure in pulmonary embolism
Preload
Management of preload, particularly fluids and diuresis, has been studied sparsely. In a study of hemodynamically stable patients with submassive PE (defined as a normotensive patient with PE and evidence of right ventricular dysfunction), the cohort receiving intravenous furosemide bolus experienced earlier improvements in parameters of right ventricular function compared with the cohort receiving volume expansion.11 More recently, in a randomized controlled trial targeting normotensive patients with intermediate-risk PE, a single high-dose bolus of furosemide improved the primary outcomes of normalization of sPESI items and reduced oligoanuria in the first 24 hours (a critical symptom of low cardiac output), and maintained stable renal function compared with placebo.12 In another trial, right ventricular dysfunction parameters did not differ between patients with intermediate high-risk PE treated with diuresis compared with volume loading; however, diuretics were tolerated safely, and brain-type natriuretic peptide was normalized earlier in the diuretic group.13
Although supporting evidence is limited, reducing the preload of an overloaded right ventricle during acute PE with diuresis would be expected to decrease right ventricular load and stress, and fluids could be detrimental. However, if a patient with acute PE has low central venous pressure (by ultrasonography of the inferior caval vein), a modest fluid challenge (≤ 500 mL) may improve the cardiac index.7 When volume overload is evident, diuresis with concomitant support from vasopressors can be pursued despite hypotension, to target improvement in cardiac output while reducing right ventricular dilation.
Afterload and reperfusion therapy
Because the thrombus drives the acute increase in right ventricular afterload that is the primary cause of right ventricular dysfunction, it is essential to plan reperfusion early in the course of care.
High-risk PE. Primary reperfusion with systemic thrombolysis remains the treatment of choice for patients with high-risk (or massive) PE (defined as syncope, systemic arterial hypotension, cardiogenic shock, or resuscitated cardiac arrest), and, together with anticoagulation initially with unfractionated heparin, readily improves pulmonary vascular resistance, pulmonary artery pressure, and obstruction.7,14 Absolute contraindications to intravenous thrombolysis include the following7:
A history of hemorrhagic stroke or stroke of unknown origin
Ischemic stroke in the previous 6 months
Central nervous system neoplasm
Major trauma, surgery, or head injury in previous 3 weeks
Bleeding diathesis
Active bleeding.
For systemic thrombolysis, lower doses of recombinant tissue–type plasminogen activator (50 mg over 2 hours compared with 100 mg over 2 hours) showed similar efficacy and possibly better safety in patients with acute PE.15
The ERS guidelines define the classes of PE treatment recommendations, with surgical embolectomy being recommended (class I indication: evidence or agreement of the benefit of treatment) for patients with high-risk PE who have contraindications to systemic thrombolysis or in whom thrombolysis has failed.7 Bayiz et al16 demonstrated the safety and efficacy of percutaneous mechanical aspiration thrombectomy in patients with massive or high-risk PE, who, at follow-up, had decreased pulmonary clot burden and improved hemodynamic parameters, pulmonary artery pressure, right ventricular end-diastolic pressure, and right ventricle-to-left ventricle ratio. Currently, percutaneous catheter-directed treatment is a class IIa indication (weight of evidence favors usefulness of treatment) and should be considered for patients with massive or high-risk PE when thrombolysis is contraindicated or has failed.7
Low- and intermediate-risk PE. Anticoagulation is recommended (class I indication) for patients with low-to intermediate-risk PE. When oral anticoagulation is initiated, direct oral anticoagulants are preferred over vitamin K antagonists,7 as these agents have a lower risk of bleeding complications and similar efficacy.17
Intermediate-risk (or submassive) PE has been a focus of research in recent years, given the risk for decompensation and subsequent right ventricular strain and dysfunction. Patients with intermediate-risk PE vary in clinical presentation,1 making management of the clot a crucial part of clinical decision-making. Thrombolysis can prevent hemodynamic decompensation and death in patients with intermediate-risk PE, but it also carries an increased risk of major hemorrhage and stroke.18 In the PEITHO (Pulmonary Embolism Thrombolysis) trial,19 thrombolysis in patients with intermediate-risk PE did not affect long-term mortality and did not reduce right ventricular dysfunction or clinical symptoms, compared with anticoagulation alone. In patients with contraindications or in whom thrombolysis has failed, surgical embolectomy was reported to have a high survival rate.20
Another modality for thrombus management is placement of an inferior caval vein filter to prevent a clot from reaching the right side of the heart and pulmonary circulation. Routine use of inferior caval vein filters is not recommended, but they should be considered in patients with absolute contraindications to anticoagulation or recurrent PE despite therapeutic anticoagulation.7
In conclusion, strategies for reperfusion treatment of intermediate-risk PE remain a gray area. The ERS guidelines7 determined that anticoagulation is the only class I recommendation for initial treatment of patients with intermediate or high clinical probability of PE. However, promising studies with a focus on emerging techniques are currently taking place.
Percutaneous interventions for clot management
Catheter-directed therapies have emerged over recent years as safe and effective options.
Catheter-directed thrombolysis. In the ULTIMA (Ultrasound Accelerated Thrombolysis of Pulmonary Embolism) trial,21 ultrasonography-assisted catheter-directed thrombolysis in patients with intermediate-risk PE, in addition to anticoagulation, resulted in reversal of right ventricular dilation and decreased mean right ventricle-to-left ventricle ratio at 24 hours compared with anticoagulation alone, without an increase in bleeding complications.
SEATTLE-II (A Prospective, Single-Arm, Multicenter Trial of EkoSonic Endovascular System and Activase for Treatment of Acute Pulmonary Embolism)22 studied 150 patients with right ventricular dysfunction (79% had submassive PE and 21% had massive PE) who underwent catheter-directed low-dose fibrinolysis. This therapy resulted in a significant decrease in mean right ventricle-to-left ventricle ratio 48 hours after the procedure (P < .0001) and reduced mean pulmonary artery systolic pressure, with a safe bleeding profile and no intracranial hemorrhage at 72 hours.22 In the OPTALYSE-PE (Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Pulmonary Embolism) trial,23 catheter-directed therapy with low-dose tissue plasminogen activator administered using a shorter duration of delivery improved clot burden and right ventricular function.
More recent studies have explored outcomes of catheter-directed therapies in patients with intermediate high-risk PE. The CANARY (Catheter-Directed Thrombolysis vs Anticoagulation in Patients With Acute Intermediate-High-Risk Pulmonary Embolism) randomized clinical trial24 showed significantly lower right ventricle-to-left ventricle ratio at the 3-month echocardiography follow-up after catheter-directed thrombolysis (P = .01) compared with anticoagulation monotherapy, although the study was prematurely terminated due to the COVID-19 pandemic. There is an ongoing study comparing catheter-directed thrombolysis vs anticoagulation alone in patients with intermediate high-risk PE.25
Catheter-directed percutaneous mechanical thrombectomy is an emerging effective treatment option in patients with high- and intermediate-risk PE, without thrombolytic complications and their associated bleeding adverse events. FLARE (FlowTriever Pulmonary Embolectomy Clinical Study)26 showed significant improvement in the right ventricle-to-left ventricle ratio 48 hours after the procedure, with minimal major bleeding, in patients with intermediate-risk PE. The recent FLAME (FlowTriever for Acute Massive PE) study27 compared peripheral mechanical thrombectomy with other contemporary therapies (systemic thrombolysis or anticoagulation alone) in patients with high-risk PE and found a lower rate of in-hospital adverse outcomes and 1.9% all-cause mortality in the thrombectomy group. These studies are an important step forward in management options other than systemic thrombolysis for hemodynamically unstable patients with high-risk PE.
Furthermore, a study using data from the largest US National Inpatient Sample database noted that catheter-based therapy (thrombolysis or mechanical thrombectomy) for patients with cancer and intermediate- or high-risk PE was associated with a lower risk of in-hospital mortality or cardiac arrest but had a high risk of bleeding.28
In the PERFECT (Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis) multicenter registry,29 101 patients with massive (high-risk) and submassive (intermediate high-risk) PE were treated with catheter-directed therapy, including mechanical or pharmacomechanical thrombectomy or thrombolysis. Clinical success—defined as hemodynamic stability, improvement in pulmonary artery pressure and right heart strain, and survival to hospital discharge—was achieved in 85.7% of patients with massive PE and 97.3% of patients with submassive PE. These therapies were found to be safe, with improved right-sided heart function.
PHARMACOLOGIC SUPPORT
Inotropes and vasopressors
In patients with high-risk PE and shock, vasopressors are often needed to maintain systemic blood pressure and improve end-organ and coronary perfusion.7 In cases of persistently low cardiac output despite vasopressor therapy, it is advisable to consider contractility support with inotropes. Norepinephrine and dobutamine are currently recommended as class IIa options for patients with high-risk PE. However, dobutamine should be used cautiously to enhance right ventricular and cardiac output, as it may worsen ventilation-perfusion mismatch and increase the risk of arrhythmias. Additionally, using dobutamine without a vasopressor can exacerbate hypotension.
Pulmonary vasodilators
The role of pulmonary vasodilation has also been explored in right ventricular dysfunction in patients with PE. In a randomized clinical trial with 20 patients who had acute intermediate high-risk PE, a single-dose of sildenafil (in 10 patients) did not significantly improve the cardiac index and instead lowered blood pressure compared with placebo.30 In another randomized clinical trial, patients with severe acute submassive (intermediate high-risk) PE who received treatment with nitric oxide did not achieve the primary composite end point of normal right ventricle on echocardiogram and normal plasma troponin T.31 However, a preplanned post hoc analysis showed that 29% more patients treated with nitric oxide had resolution of right ventricular dilation or hypokinesis at 24 hours.31 In a randomized clinical trial of 14 patients with acute PE (hemodynamically stable with high clinical probability of right ventricular dysfunction), epoprostenol compared with placebo did not result in improvement of right ventricular dilation or other parameters.32
While pulmonary vasodilators can be beneficial in specific types of pulmonary hypertension, their effectiveness in right ventricular failure associated with pulmonary vascular issues may be limited. This is likely due to the unique pathophysiology of PE, particularly the acute increase in afterload resulting from a substantial clot burden. Overall, pulmonary vasodilators are not encouraged, as they can aggravate hypoperfusion of organs and systemic hypotension, despite efforts to decrease blood pressure and pulmonary vascular resistance.7
SUPPORTIVE CARE
For hypoxemic patients, prompt administration of supplemental oxygen therapy is essential. Intubation and mechanical ventilation are reserved for select patients with refractory hypoxemia and unstable hemodynamics, with careful use of anesthetic agents to avoid hypotension and cautious monitoring given the detrimental effects of positive end-expiratory pressure.7
In recent years, an integrated approach involving multidisciplinary teams, early risk stratification, and prompt decision-making in patients with intermediate-risk PE has led to lower rates of all-cause mortality,33 reduced intensive care unit and overall hospital length of stay,34 and high survival-to-discharge rates.35 A multidisciplinary team approach in patients with high-risk PE and certain patients with intermediate-risk PE is a class IIa recommendation.7 In the long term, if patients develop pulmonary hypertension as a complication, referral to a pulmonary hypertension center is highly recommended.
MECHANICAL CIRCULATORY SUPPORT AND ADVANCED THERAPY
Extracorporeal membrane oxygenation
Some patients may experience clinical deterioration that is resistant to treatment, including cardiac arrest or worsening hemodynamic shock requiring increased vasopressor support. In these cases, extracorporeal membrane oxygenation (ECMO) can be used as a bridging or rescue therapy; the ERS classified this as a class IIb recommendation (efficacy of treatment is less well established).7 Venoarterial ECMO can bypass blocked pulmonary circulation by providing sufficient cardiac output to sustain systemic and coronary blood flow until the thrombus is effectively managed. However, using ECMO for an extended period, typically more than 5 to 10 days, can result in complications.7
Another detailed analysis from the National Inpatient Sample database of patients with high-risk PE revealed that use of ECMO in patients with massive PE increased from 0.07% to 1.1% from 2005 to 2013 and its use was not associated with a change in in-hospital mortality (61.6%).36 ECMO was performed in 0.3% of hospitalized patients with high-risk PE for a duration of 1.9 ± 4.1 days from the index admission date, and the median hospital length of stay was 10 days. In patients with high-risk PE using ECMO for hemodynamic support, independent predictors of mortality included age, female sex, obesity, congestive heart failure, and chronic pulmonary disease.36
A handful of studies have looked at using ECMO as a bridge to ultimate clot management in patients with high-risk PE. In a cohort of 20 patients with high-risk PE who were managed with venoarterial ECMO for a median 5.1 days, 94.7% had normal right ventricular function at discharge.37 Another group of 16 patients with acute high-risk PE, 12 of whom were in cardiac arrest, underwent venoarterial ECMO for a mean duration of 1.5 days and had an overall 30-day mortality rate of 43.8%; treatment was mainly with ECMO alone, ECMO with thrombolysis, and ECMO with embolectomy.38 While a meta-analysis on venoarterial ECMO and acute massive PE did not show significantly different in-hospital mortality between patients treated with or without ECMO,39 another meta-analysis of venoarterial ECMO showed low-quality evidence of higher survival rates in patients 60 years or younger and in those who underwent surgical embolectomy.40 There was evidence that venoarterial ECMO improves short-term survival of patients with acute PE.40
The 2022 American Heart Association guidelines maintain their earlier statement on cardiopulmonary resuscitation, which suggests that extracorporeal cardiopulmonary resuscitation for cardiac arrest from PE can be considered as a bridge to reperfusion therapy in select patients when it can be implemented and conventional cardiopulmonary resuscitation is failing.41
Right ventricular assist devices
Right ventricular assist devices have been explored for refractory shock related to PE. The devices can be placed surgically, with cannulation performed in the right atrium or right ventricle and pulmonary artery while connected with an extracorporeal flow pump.4 They also can be placed percutaneously with lower flow than surgical right ventricular assist devices. Right ventricular assist devices, while offloading the right atrium and right ventricle from excessive preload, ultimately increase right ventricular afterload by generating constant flow and pressure in the pulmonary artery.4 The percutaneous right ventricular assist device Impella RP, when used in hemodynamically unstable patients with PE, can lead to shock reversal with improvement in cardiac index and hemodynamic stability.10
Overall, there is a lack of sufficient evidence on outcomes of patients with acute high-risk PE undergoing treatment with ECMO, Impella RP, or other right ventricular assist devices. The decision to bridge with mechanical circulatory support should be made on a case-by-case basis, using a multidisciplinary team approach that involves intensivists, cardiologists with expertise in heart failure, pulmonologists, and cardiothoracic surgeons.
CONCLUSION
The incidence and burden of PE have risen in recent decades. Mortality, particularly in patients at high-risk with significant right ventricular involvement, is alarmingly high. An integrated approach to risk stratification and prompt implementation of therapies are critical to manage acute, life-threatening and long-term sequelae of PE, given PE’s relationship with right-sided heart dysfunction. There are evidence gaps in the management of right ventricular failure due to PE, and further studies are warranted to aid in decision-making.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2025 The Cleveland Clinic Foundation. All Rights Reserved.