If a patient experiences diarrhea, hematochezia, or abdominal pain within the first 6 weeks of therapy with one of the anticancer drugs known as immune checkpoint inhibitors (ICIs), the first step is to rule out infection, especially with Clostridium difficile. The next step is colonosocopy with biopsy or computed tomography.
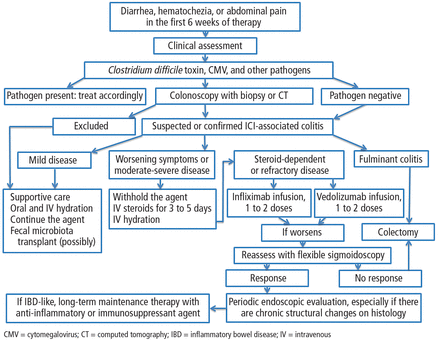
Patients with mild ICI-associated colitis may need only supportive care, and the ICI can be continued. In moderate or severe cases, the agent may need to be stopped and corticosteroids and other colitis-targeted agents may be needed. Figure 1 shows our algorithm for diagnosing and treating ICI-associated colitis.
Proposed diagnosis and management of immune checkpoint inhibitor (ICI)-associated colitis.
POWERFUL ANTICANCER DRUGS
ICIs are monoclonal antibodies used in treating metastatic melanoma, non-small-cell lung cancer, metastatic prostate cancer, Hodgkin lymphoma, renal cell carcinoma, and other advanced malignancies.1,2 They act by binding to and blocking proteins on T cells, antigen-presenting cells, and tumor cells that keep immune responses in check and prevent T cells from killing cancer cells.1 For example:
Ipilimumab blocks cytotoxic T lymphocyte-associated antigen 4
Nivolumab and pembrolizumab block programmed cell death protein 1
Atezolizumab blocks programmed death ligand 1.1
With these proteins blocked, T cells can do their job, often producing dramatic regression of cancer. However, ICIs can cause a range of immune-related adverse effects, including endocrine and cutaneous toxicities, iridocyclitis, lymphadenopathy, neuropathy, nephritis, immune-mediated pneumonitis, pancreatitis, hepatitis, and colitis.3
ICI-ASSOCIATED COLITIS IS COMMON
ICI-associated colitis is common; it is estimated to affect about 30% of patients receiving ipilimumab, for example.4 Clinical presentations range from watery bowel movements, blood or mucus in the stool, abdominal cramping, and flatulence to ileus, colectasia, intestinal perforation, and even death.5
The incidence appears to increase with the dosage and duration of ICI therapy. The onset of colitis typically occurs 6 to 7 weeks after starting ipilimumab,6 and 6 to 18 weeks after starting nivolumab or pembrolizumab.7 Table 1 lists the incidence of diarrhea and colitis and time of onset to colitis with common ICIs. However, colitis, like other immune-related adverse events, can occur at any point, even after ICI therapy has been discontinued.8
Colitis with common immune checkpoint inhibitors: Incidence of diarrhea and colitis, and time to onset
It is best to detect side effects of ICIs promptly, as acute inflammation can progress to chronic inflammation within 1 month of onset.9 We believe that early intervention and close monitoring may prevent complications and the need for long-term immunosuppressive treatment.
Patients, family members, and caregivers should be informed of possible gastrointestinal along with systemic side effects. Severe gastrointestinal symptoms such as increased stool frequency and change in stool consistency should trigger appropriate investigation and the withholding of ICI therapy.
COLITIS IS A SPECTRUM
The colon appears to be the gastrointestinal organ most affected by ICIs. Of patients with intestinal side effects, including diarrhea, only some develop colitis. The severity of ICI-associated colitis ranges from mild bowel illness to fulminant colitis.
Hodi et al,10 in a randomized trial in which 511 patients with melanoma received ipilimumab, reported that approximately 30% had mild diarrhea, while fewer than 10% had severe diarrhea, fever, ileus, or peritoneal signs. Five patients (1%) developed intestinal perforation, 4 (0.8%) died of complications, and 26 (5%) required hospitalization for severe enterocolitis.
The pathophysiology of ICI-mediated colitis is unclear. Most cases are diagnosed clinically.
Colitis is graded based on the Montreal classification system11:
Mild colitis is defined as passage of fewer than 4 stools per day (with or without blood) over baseline and absence of any systemic illness.
Moderate is passage of more than 4 stools per day but with minimal signs of systemic toxicity.
Severe is defined as passage of at least 6 stools per day, heart rate at least 90 beats per minute, temperature at least 37.5°C (99.5°F), hemoglobin less than 10.5 g/dL, and erythrocyte sedimentation rate at least 30 mm/h.11
RULE OUT INFECTION
If symptoms such as diarrhea or abdominal pain arise within 6 weeks of starting ICI therapy, then we should check for an infectious cause. The differential diagnosis of suspected ICI-associated colitis includes infections with C difficile, cytomegalovirus, opportunistic organisms, and other bacteria and viruses. ICI-induced celiac disease and immune hyperthyroidism should also be ruled out.4
CONSIDER COLONOSCOPY AND BIOPSY
Once infection is ruled out, colonoscopy should be considered if symptoms persist or are severe. Colonoscopy with biopsy remains the gold standard for diagnosis, and it is also helpful in assessing severity of mucosal inflammation and monitoring response to medical treatment.
Table 2 lists common endoscopic and histologic features of ICI-mediated colitis; however, none of them is specific for this disease.
Immune checkpoint inhibitor-associated colitis: Common endoscopic and histologic features
Common endoscopic features are loss of vascular pattern, edema, friability, spontaneous bleeding, and deep ulcerations.12 A recent study suggested that colonic ulcerations predict a steroid-refractory course in patients with immune-mediated colitis.4
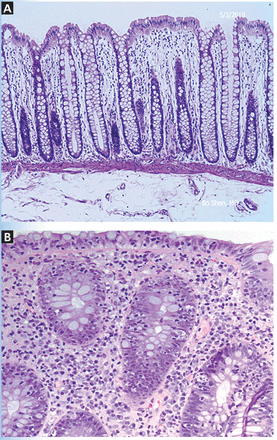
Histologically, ICI-associated colitis is characterized by both acute and chronic changes, including an increased number of neutrophils and lymphocytes in the epithelium and lamina propria, erosions, ulcers, crypt abscess, crypt apoptosis, crypt distortion, and even noncaseating granulomas.13 However, transmural disease is rare. Figure 2 compares the histopathologic features of ICI-associated colitis and a normal colon.
Histologic features of immune checkpoint inhibitor-associated colitis. High-resolution images of the colon showing normal histopathology (A), and colonic mucosa with intraepithelial lymphocytosis and occasional apoptosis in crypt epithelium (B) (hematoxylin and eosin, × 200).
COMPUTED TOMOGRAPHY CAN BE USEFUL
Computed tomography (CT) can also be useful for the diagnosis and measurement of severity.
Garcia-Neuer et al14 analyzed 303 patients with advanced melanoma who developed gastrointestinal symptoms while being treated with ipilimumab. Ninety-nine (33%) of them reported diarrhea during therapy, of whom 34 underwent both CT and colonoscopy with biopsy. CT was highly predictive of colitis on biopsy, with a positive predictive value of 96% and a negative likelihood ratio of 0.2.14
TREATMENT
Supportive care may be enough when treating mild ICI-related colitis. This can include oral and intravenous hydration4 and an antidiarrheal drug such as loperamide in a low dose.
Corticosteroids. For moderate ICI-associated colitis with stool frequency of 4 or more per day, patients should be started on an oral corticosteroid such as prednisone 0.5 to 1 mg/kg per day. If symptoms do not improve within 72 hours of starting an oral corticosteroid, the patient should be admitted to the hospital for observation and escalation to higher doses or possibly intravenous corticosteroids.
Infliximab has been used in severe and steroid-refractory cases,13 although there has been concern about using anti-tumor necrosis factor (TNF) agents such as this in patients with malignancies, especially melanoma. Since melanoma can be very aggressive and anti-TNF agents may promote it, it is prudent to try not to use this class of agents.
Other biologic agents such as vedolizumab, a gut-specific anti-integrin agent, are safer, have theoretic advantages over anti-TNF agents, and can be considered in patients with steroid-dependent or steroid-refractory ICI-associated enterocolitis. A recent study suggested that 2 to 4 infusions of vedolizumab are adequate to achieve steroid-free remission.15 Results from 6 clinical trials of vedolizumab in 2,830 patients with Crohn disease or ulcerative colitis did not show any increased risk of serious infections or malignancies over placebo.16,17 A drawback is its slow onset of action.
Surgery is an option for patients with severe colitis refractory to intravenous corticosteroids or biological agents, as severe colitis carries a risk of significant morbidity and even death. The incidence of bowel perforation leading to colectomy or death in patients receiving ICI therapy is 0.5% to 1%.18,19
Fecal microbiota transplant was associated with mucosal healing after 1 month in a case report of ICI-associated colitis.9
Follow-up. In most patients, symptoms resolve with discontinuation of the ICI and brief use of corticosteroids or biological agents. Patients with recurrent or persistent symptoms while on long-term ICI therapy may need periodic endoscopic evaluation, especially if there are chronic structural changes on histologic study.
If patients have recurrent or persistent symptoms along with chronic inflammatory structural changes on histology, a sign of an inflammatory bowel diseaselike condition, long-term maintenance therapy with an anti-inflammatory or immunosuppressant agent may be considered. However, there is no consensus on the treatment of this condition. It can be treated in the same way as classic inflammatory bowel disease in the setting of concurrent or prior history of malignancy, especially melanoma. Certain agents used in inflammatory bowel disease such as methotrexate and vedolizumab carry a lower risk of malignancy than anti-TNF agents and can be considered. A multidisciplinary approach that includes an oncologist, gastroenterologist, infectious disease specialist, and colorectal surgeon is imperative.
- Copyright © 2018 The Cleveland Clinic Foundation. All Rights Reserved.