ABSTRACT
Capsule endoscopy, also known as wireless capsule endoscopy or video capsule endoscopy, is a noninvasive procedure that uses a swallowed capsule-shaped miniature camera for direct visual and diagnostic evaluation of gastrointestinal (GI) disease. Although originally intended as a tool to examine the small intestine, which is mostly beyond the reach of conventional endoscopy, capsule endoscopy is now also being used to examine the entire length of the GI tract.
Currently, capsule endoscopy is useful in diagnosing occult GI bleeding in the small bowel, celiac disease, Crohn disease, small-bowel tumors, and nonsteroidal anti-inflammatory drug-induced enteropathy in the small and large bowel.
Capsule endoscopy is not recommended as a routine screening tool for colon cancer.
Sedatives and pain medications are not required for capsule endoscopy, essential medications need not be withheld on the day of studies, and patients can go about their usual activities of daily living.
A significant drawback of capsule endoscopy is the inability to fully control the movement of the device, perform suction, or obtain a biopsy.
In the future, endoscopic capsules will allow obtaining biopsy samples, analyzing and coagulating lesions, and even treating them.
Capsule endoscopy was conceived as a tool to examine the small intestine, which is beyond the reach of conventional endoscopes, but it is now being used to diagnose and monitor diseases throughout the entire gastrointestinal (GI) tract. Although conventional endoscopy remains the gold standard for looking at the esophagus and stomach proximally and the colon distally, capsule endoscopy has the advantage of being noninvasive.
Thus, although capsule endoscopy is mainly used to look for sources of obscure bleeding in the small bowel, it is also being used in the evaluation and diagnosis of celiac disease, Crohn disease, small-bowel tumors, nonsteroidal anti-inflammatory drug (NSAID)-induced enteropathy, and esophageal, gastric, and large-bowel diseases. It is also used in monitoring treatment outcomes. A limitation of current capsules is that they cannot perform inflation, suction, or biopsy.
In this article, we review the current role of capsule endoscopy in the evaluation, diagnosis, and treatment of GI disease in adults.
CHALLENGES OF EXAMINING THE SMALL INTESTINE
The GI tract is about 30 feet (9 m) long from mouth to anus. The small intestine makes up about two-thirds of the entire tract and is subject to diseases such as bleeding, ulcers, malabsorption, inflammation, strictures, polyps, and cancer.
The GI tract can be examined indirectly by radiologic studies such as contrast barium swallow, small-bowel follow-through, and barium enema, but these offer no opportunity to obtain biopsy specimens or perform treatment. On the other hand, fiberoptic upper endoscopy, colonoscopy, and enteroscopy (“push enteroscopy,” for part of the small intestine) offer direct visualization.
But it is not possible to examine the entire small intestine with routine endoscopy. Single-balloon, double-balloon, and spiral endoscopes have been invented for this purpose, but it can be a challenge even with antegrade and retrograde balloon enteroscopic techniques. Further, these techniques usually require general anesthesia, long procedure times, advanced skills, and fluoroscopy, which exposes the patient to radiation. Therefore, the need to directly visualize the entire small intestine led to the birth of the capsule endoscope.
INVENTION OF THE CAPSULE ENDOSCOPE
Capsule endoscopy began in 1981, when Gavriel Iddan, an engineer from the Israeli Defense Ministry, collaborated with gastroenterologist Eitan Scapa while both were on sabbatical studies in Boston, MA. Further collaboration with Paul Swain, an English gastroenterologist, and Shuji Nakamura’s invention of the light-emitting diode as a light source for optical devices (for which he won the Nobel Prize in 2014) led to further refinement of the capsule endoscope for evaluating small-bowel disease.1
The basic components of capsule endoscopy are the following:
The capsule, containing one or more cameras, a light source, battery, and transmitter
In most systems, sensors placed on the surface of the patient’s abdomen similar to electrocardiographic leads on the chest, or contained in a belt worn by the patient that is connected to a recorder
Software to process and display images to be reviewed by a physician.
Devices have improved over the years, with wider fields of view (140°–360°), more cameras (up to 4 in some models), longer battery life, and variable frame rates so that the capsule can take as few as 2 frames (pictures) per second when traveling slowly through the stomach and intestines and up to 35 per second when traveling quickly through the distal esophagus.
In its journey through the GI tract, the capsule can acquire 50,000 to 60,000 images, which can take from 30 to 90 minutes to review. Software allows for a quick preliminary review and single or group viewing of 2 or 4 images. Real-time viewing is particularly important in detecting active GI bleeding.
Detailed reports of the procedure should be generated and at a minimum include patient demographic information, the indication for the procedure, type of device used, the diagnosis based on findings, and management recommendations.
EXPANDING ROLES FOR CAPSULE ENDOSCOPY
Current indications for capsule endoscopy of the small intestine in adults (Table 1) include diagnosis of obscure GI bleeding and chronic iron-deficiency anemia, small-bowel tumors, and NSAID-induced enteropathy, and diagnosis and assessment of treatment outcomes of celiac and Crohn disease. It is also used in screening for and surveillance of familial adenomatous polyposis syndrome, Barrett esophagus, and esophageal varices. In addition, there is interest in using capsule endoscopy to manage acute GI bleeding, both in the emergency department to help in deciding whether the patient needs to be admitted to the hospital, and after admission to the hospital.
Current indications for capsule endoscopy in adults
In the large bowel, capsule endoscopy is indicated only in cases when colonoscopy was started but could not be completed and in patients at moderate to high risk from sedation or severe cardiopulmonary conditions. It is not for routine colon cancer screening.
Capsule endoscopy has multiple contraindications that include cognitive impairment, risk factors for capsule retention, and active Crohn disease (Table 2). It should be used with caution in patients who have cardiac implantable electronic devices such as pacemakers, automatic implantable cardiac defibrillators, or left ventricular assist devices. These devices can possibly cause electromagnetic interference that can produce image artifacts, but the capsule does not affect the cardiac device.2
Contraindications to capsule endoscopy for small-bowel disease
Chief advantages of capsule endoscopy are that it is noninvasive, does not require sedation, and does not require that essential medications be withheld on the day of the procedure (Table 3). Images are of high quality, with 1:8-fold magnification, which can show individual villi (Figure 1a).3 A drawback is the lack of suctioning, flushing, or biopsy capability, and therapeutic interventions are not possible with current devices. In addition, results may be prone to overinterpretation by readers.4 Its learning curve is steep: accuracy is higher in diagnosing prominent intraluminal lesions, active bleeding ulcers, tumors, and stenosis, and lower with subtle lesions, erosions, angiodysplasias (also called angioectasias), and diverticula (Figure 1b).3,5
Advantages of capsule endoscopy
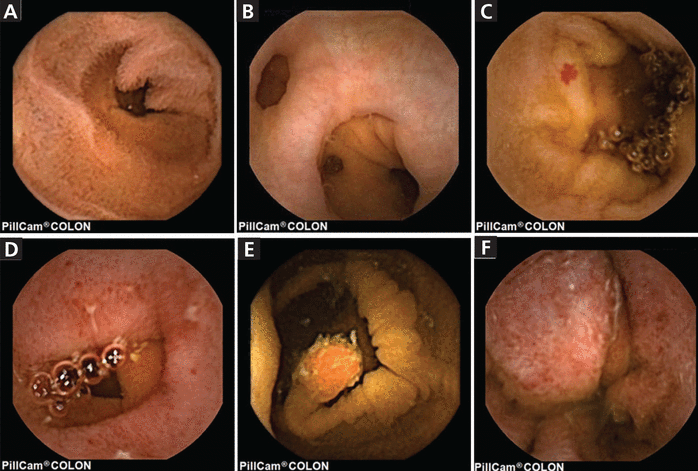
Capsule endoscopy of the small bowel. A, normal; B, diverticula; C, angiodysplasia; D, erosions of Crohn disease; E, a 10-mm pedunculated polyp; and F, ulcerative colitis.
Used with permission of Annals of Translational Medicine, from Toth E, Yung DE, Nemeth A, Johansson GW, Thorlacius H, Koulaouzidis. A Video capsule colonoscopy in routine clinical practice. Ann Transl Med 2017; 5(9):195. doi:10.21037/atm.2017.03.91. Permission conveyed through Copyright Clearance Center Inc.
A consensus committee of the Canadian Association of Gastroenterology has offered a list of recommendations for and against capsule endoscopy for various conditions (Table 4).6
Canadian Association of Gastroenterology consensus recommendations for and against capsule endoscopya
CAPSULE ENDOSCOPY IN SMALL-BOWEL DISEASE
Capsule endoscopy has evolved to the point that different capsules are available to examine different parts of the GI tract. Capsules optimized for the small intestine include the following:
PillCam SB3 (Given Imaging), a third-generation device that weighs less than 4 g, measures 11 mm by 26 mm, has higher resolution than earlier models, and has variable frame rates—up to 6 frames per second when going through fast areas such as the duodenum, and down to 2 frames per second when stationary or moving slowly. It also has a blood detector to help identify sites of bleeding.
Olympus Endocapsule 10 (Olympus), which measures 11 mm by 26 mm, weighs 3.3 g, has a 160-degree wide-view camera with 4 lights, takes 2 frames per second, takes higher-resolution pictures for better clarity, and provides software-mediated 3-dimensional views of the small intestine. Both PillCam SB3 and Endocapsule 10 have battery lives of about 8 to 12 hours.
Micro Cam (IntroMedic) and OMOM (Jinshan Science & Technology) are other capsule endoscopes used worldwide.
All small-bowel capsule endoscopes have comparable diagnostic yields.7
Obscure gastrointestinal bleeding and chronic iron-deficiency anemia
GI bleeding is called obscure when traditional upper and lower endoscopy and radiography fail to find the source. It is the most common reason capsule endoscopy is performed. Of note, GI bleeding can be both obscure and overt, ie, manifested clinically by hematemesis (vomiting blood), hematochezia (blood in stool), and melena (black stool).
Bleeding from the small bowel is uncommon, accounting for only 5% to 10% of cases of GI bleeding, but it is responsible for up to 80% of cases of obscure GI bleeding and can manifest as iron-deficiency anemia.8 Most of these lesions are angiodysplasias (Figure 1c),3 which are tufts of abnormal vessels caused by abnormal connections between arteries and veins that bypass the capillary system. They are small, bleed intermittently, and share similarities with other angiodysplasias that have never bled.9–11
In a study of 911 patients who underwent evaluation for obscure GI bleeding,11 capsule endoscopy found the source of the obscure bleeding in 509 (56%) of the 911 patients: 203 patients (22%) had small-bowel disease, 88 (10%) had ulcerations, 70 (8%) had tumors, 24 (3%) had varices, and 73 (8%) had blood in the small bowel with no lesions identified. Lesions of the esophagus or stomach were found in 97 patients (11%).11
A systematic review of 22,840 procedures in 227 studies of capsule endoscopy12 found a detection rate of 61% for obscure small-bowel bleeding. Angiodysplasia was the most common cause.
The timing of capsule endoscopy is important in detecting the source of obscure GI bleeding, as the detection rate decreases with time: 55% within 1 day of admission vs 18% 5 days after admission.13
The cause of obscure GI bleeding sometimes goes undiagnosed even with capsule endoscopy. But the chances of rebleeding in such cases appears to be low and does not warrant further invasive investigations.14
Celiac disease
Celiac disease is an autoimmune enteropathy characterized by an immunologic response to gluten, which is ubiquitous in food and additives; it usually responds to a gluten-free diet. Diagnosis relies on symptoms (eg, chronic diarrhea, bloating, abdominal discomfort), characteristic biomarkers, histologic analysis, and response to a gluten-free diet. Screening tests include tissue transglutaminase antibodies and deamidated gliadin peptide antibodies.
The diagnostic test of choice is upper-GI endoscopy with duodenal biopsy. Celiac disease causes architectural changes in the villi, and villous atrophy is common.15–17 Capsule endoscopy can show villous atrophy in its magnified images. However, villous atrophy can also be seen in conditions such as Crohn disease, lymphoma, amyloidosis, human immunodeficiency virus infection, food allergies, drugs, and chemotherapy.18
Capsule endoscopy is indicated in patients who have positive serology or symptoms suggestive of celiac disease but are unable or unwilling to undergo routine upper endoscopy. It is also indicated in patients with normal duodenal histology to identify distal small-bowel lesions. In patients with celiac disease, it helps detect serious complications such as ulcerative jejunoileitis, celiac-associated lymphoma, and adenocarcinoma, and can be used to monitor unexplained symptoms due to inadvertent gluten use, which is seen in about 48% of patients with nonresponsive celiac disease.19,20
Capsule endoscopy is also of value when clinical symptoms are highly suspicious for celiac disease despite negative serology, as is seen in 5% of patients.19
Crohn disease
Crohn disease is an inflammatory bowel disease that can affect any part of the GI tract from the mouth to the anus. About 50% of patients have disease in the colon and terminal ileum, 30% in the small bowel, and 20% in the colon only.21 The disease is complicated by fistulas, strictures, obstruction, and risk of colonic malignancies. The diagnosis is based on clinical, biochemical, radiologic, histologic, and endoscopic findings.
Characteristic symptoms of Crohn disease include diarrhea or abdominal pain for more than 6 weeks, low-grade fever, weight loss, and fatigue. Laboratory signs include elevated C-reactive protein, elevated erythrocyte sedimentation rate, elevated fecal calprotectin, anemia, and hypoalbuminemia.22
Computed tomographic (CT) enterography with small-bowel follow-through and magnetic resonance enterography can aid in determining the location and extent of Crohn disease. The characteristic findings include segmental mural hyperenhancement, wall-thickening, intramural edema, strictures, and ulcerations. But direct ileocolonoscopy remains the gold standard because it directly visualizes the intestinal mucosa and can be used to obtain a biopsy. Crohn disease is suggested endoscopically by mucosal inflammation, ulceration (linear or aphthous), cobblestone appearance of the mucosa, and stenosis.
Capsule endoscopy can detect Crohn disease through 3 findings: mucosal inflammation, disease extension, and strictures (Figure 1d).3 Difficulty in describing the lesions with capsule endoscopy has led to the development of 2 scoring systems, the Lewis score23 and the Capsule Endoscopy Crohn’s Disease Activity Index.24
Capsule endoscopy can detect disease in the terminal ileum with a sensitivity approaching 100%, superior to that of CT enterography or magnetic resonance enterography,25 and it can detect jejunal lesions, which have a high risk of relapse.26
Therefore, capsule endoscopy is recommended in patients with known, suspected, or relapsed Crohn disease with unexplained symptoms when ileocolonoscopy and imaging studies are negative or when reassessment is beyond the reach of ileocolonoscopy. It is also of value in Crohn disease recurrence or progression after small-bowel colectomy.6
Small-bowel tumors
The small bowel is the site of 2% of all gastrointestinal tumors and 10% of occult gastrointestinal bleeding. Benign tumors include leiomyoma, adenoma, lipoma, and hemangioma, while malignant lesions include adenocarcinoma, neuroendocrine tumors, gastrointestinal stromal tumors, and lymphoma. The most common sites in the small bowel are the ileum, followed by the duodenum and jejunum.27 Signs of small-bowel tumors on capsule endoscopy include protruding masses, mucosal disruption, irregular surfaces, discolored areas, and white villi. Capsule endoscopy is less sensitive in detecting small-bowel tumors in the duodenum because of anatomic variability (bulges can be mistaken for masses) and rapid transit of the capsule (preventing adequate visualization). Also, the ampulla of Vater has a higher frequency of malignant transformation, but capsule endoscopy visualizes it poorly and cannot distinguish adenomas from anatomic variations around that site.28
Intestinal polyposis syndromes are rare and include familial adenomatous polyposis and hamartomatous polyposis syndromes such as Peutz-Jeghers syndrome. When intestinal polyposis syndromes are diagnosed early, outcomes are better with surveillance, and capsule endoscopy can be very useful in this function, as it is noninvasive and obtains images of the entire small intestine.29,30
NSAID-induced enteropathy
Adverse effects of NSAIDs include abdominal pain, nausea, indigestion, bleeding, constipation, and abdominal distention. The broad spectrum of pathology of NSAID-induced enteropathy includes petechiae, reddened folds, denuded mucosa, mucosal breaks, angiodysplasias, and strictures from chronic use. Multiple ulcers and lesions are common with both acute and chronic NSAID use, even with low doses and enteric-coated preparations.
In a study in which 40 healthy volunteers underwent capsule endoscopy at baseline and then took the NSAID diclofenac for 2 weeks, repeat capsule endoscopy showed new lesions in 27 (68%).31
There are no biomarkers of NSAID-induced enteropathy. The diagnosis is based on a history of NSAID use in the previous month, an endoscopic finding of mucosal damage, and improvement of clinical course after stopping the drug in the absence of other inflammatory bowel diseases. Findings on capsule endoscopy include ulcers, erosions, scar formations, luminal stenosis, and diaphragmatic disease of the intestine (characterized by multiple thin, concentric, diaphragm-like strictures in the large and small intestine, which are pathognomonic of NSAID damage).
Evaluation of chronic abdominal pain and diarrhea
Chronic abdominal pain and diarrhea are common reasons for healthcare visits and are mostly caused by irritable bowel syndrome or functional dyspepsia.32 The role of capsule endoscopy of the small bowel in evaluating chronic abdominal pain and diarrhea was highlighted in a meta-analysis of 21 studies in 1,520 patients by Xue et al.33 The pooled diagnostic yield was 21%, but in those patients with known underlying inflammatory conditions the yield was 78%.
Biomarkers of inflammation such as erythrocyte sedimentation rate and C-reactive protein increase the yield of capsule endoscopy in finding the cause of chronic abdominal pain and diarrhea.34 Capsule endoscopy is therefore not recommended in the evaluation of patients who have chronic abdominal pain or diarrhea but no supportive positive biomarkers such as C-reactive protein.
Infiltrative diseases such as lymphoma, adenocarcinoma, amyloidosis, or sarcoidosis have no pathognomonic features on capsule endoscopy, and tissue biopsies are necessary to diagnose them.
CAPSULE ENDOSCOPY OF THE ESOPHAGUS AND STOMACH
Neoplastic and nonneoplastic diseases in the upper-GI tract have been evaluated with barium studies, fluoroscopy, CT, and magnetic resonance imaging. Conventional upper-GI endoscopy remains the diagnostic tool of choice in evaluating these diseases, and the yield is increased with the use of endoscopic ultrasonography. While capsule endoscopy is noninvasive and appeared attractive, earlier capsule devices could not adequately visualize the distal esophagus because they moved through it too quickly. This has been improved with newer capsule devices such as the following:
PillCam UGI (Medtronic) has a battery life of 90 minutes, a variable frame rate of 1 to 35 frames per second, and 2 cameras at each end.
CapsoCam (CapsoVision) has 4 cameras to provide 360° views, a battery life of 15 hours, and an embedded recorder that eliminates the need for external receiver equipment. The patient retrieves the capsule from the stool with a magnetic wand supplied in a special kit and brings or mails it back to the clinic to be uploaded and interpreted.35
Both devices are used to evaluate the esophagus, stomach, and small bowel.
Capsule endoscopy is used in diagnosing various diseases in the esophagus, such as ulcers, varices, and Barrett esophagus, which is a risk factor for esophageal cancer if left untreated. However, although capsule endoscopy is useful in screening for Barrett esophagus, it is not cost-effective in diagnosing and treating it compared with direct visualization with fiber-optic endoscopy.36
The role of capsule endoscopy in diagnosing gastric lesions is limited because of the large surface area of the stomach and the inability to control the capsule’s movements as it tumbles, even with tedious patient positional strategies.
Esophageal varices and other bleeding complications
Esophageal varices and bleeding are complications of portal hypertension and decompensated liver disease. Traditional evaluation of varices is with fiberoptic endoscopy, which allows for concurrent treatment with ligation, banding, and sclerotherapy.
The potential role of capsule endoscopy in managing varices was noted in a meta-analysis of 1,328 patients.37 The diagnostic accuracy was 90%, pooled sensitivity 83%, and specificity 85%. The authors concluded that capsule endoscopy could not replace upper endoscopy as the initial procedure of choice for patients with varices or variceal bleeding, but that it may have a role if patients decline or cannot undergo upper-GI endoscopy.
Capsule endoscopy in the emergency department
Capsule endoscopy has been studied in patients with acute upper-GI bleeding in the emergency department to determine the need for hospital admission. In a study of 71 patients,38 30 (81%) of the 37 patients initially evaluated with capsule endoscopy were discharged from the emergency department, while all patients who were not evaluated with capsule endoscopy were admitted to the hospital. Rates of recurrent bleeding and death at 30 days were similar between the 2 groups
These findings have significant cost implications and suggest that capsule endoscopy may help screen patients with GI bleeding before they are admitted to the hospital. Although it increases the length of stay in the emergency department, it reduces overall hospital costs by decreasing hospital admissions and length of stay in the hospital.
Capsule endoscopy is also being used to look for the source of bleeding after admission to the hospital. A randomized trial39 in patients admitted to the hospital because of GI bleeding without hematemesis found a 64% detection rate of the source of bleeding with early capsule endoscopy compared with 31% with routine care (standard direct upper endoscopy followed later by other studies). There were no differences in mortality or rebleeding rates between the 2 groups after discharge. However, early capsule endoscopy detected more vascular lesions such as angiodysplasias (19% compared with 4.4% in the routine-care group). Angiodysplasias are characteristically small, bleed intermittently, and do not leave any mucosal footprints of recent bleeding, and those lesions that bleed have a higher rate of rebleeding.39
CAPSULE ENDOSCOPY OF THE COLON
Colonic capsule endoscopes include the following:
PillCam Colon (Given Imaging) has 2 cameras, one at each end. It is about 5 mm longer than the small-bowel capsule, guaranteeing that the entire surface of the colon is examined with a wide angle of view of 172° per camera. The capsule frame rate is adjustable, from 4 frames per second to 35 frames per second, and can take up to 30,000 pictures before the battery runs out. It has 2 approved indications from the US Food and Drug Administration. One indication is for incomplete colonoscopy, which occurs in about 5% of the 14 million colonoscopies each year. The other indication is detection of colon polyps in patients with evidence of lower-GI bleeding in whom colonoscopy or moderate sedation would pose major risks, but in whom colonoscopy can still be tolerated if a clinically significant abnormality is found.
PillCam Crohn’s Capsule has dedicated rapid software and the ability to detect subtle mucosal lesions. The small bowel is divided into 3 segments (tertiles) and scored using the Lewis score.23 The left and right colon are similarly scored for severity and extent of disease, strictures, and response to treatment.
Colorectal cancer
Colorectal cancer is the third most common cause of cancer-related death in men (after lung and prostate cancer) and also the third most common cause in women (after lung and breast cancer). About 148,000 new cases are diagnosed yearly, 105,000 in the colon and 43,000 in the rectum.40
When colorectal cancer is diagnosed at an early stage, the 5-year survival rate is 90%. Screening tools include flexible sigmoidoscopy, CT colonography, and fecal testing, but colonoscopy remains the gold standard diagnostic test, with its ability to survey the entire colon, obtain tissue biopsies, and remove polyps. Patients often put off or avoid colonoscopy because they perceive it to be invasive, embarrassing, and uncomfortable, and because it requires long bowel preparation, sedation, and often pain medication, and because it poses risks of perforation, bleeding, and cardiopulmonary complications.
CT colonoscopy (virtual colonoscopy) is an outpatient imaging procedure that can take from 30 to 60 minutes and requires air or carbon dioxide inflation of the colon for optimal visualization of colonic mucosa. It does not require anesthesia. However, traditional colonoscopy is needed if a lesion is found or if incidental findings warrant further testing, and it exposes the patient to ionizing radiation.
Capsule endoscopy is less invasive. Although sedation is not needed, the colonic cleansing preparation must be close to perfect to provide excellent images since it has no suctioning or flushing capability. Bowel preparation with 4 L of polyethylene glycol solution in split doses in the evening and morning before the procedure or with a low-volume sodium phosphate agent is sometimes employed for better preparation and for enhanced movement of the device.
Colonic capsule endoscopy was used for polyp detection in a prospective study of 884 patients without symptoms who underwent this procedure followed by conventional colonoscopy. Capsule endoscopy had a sensitivity of 81% and a specificity of 93% for polyps 6 mm or larger, and a sensitivity of 80% and specificity of 97% for polyps 10 mm or larger (Figure 1e).3 Serrated polyps accounted for 25% of missed polyp diagnoses.41
Other uses
In a study of cases of incomplete colonoscopy in which capsule endoscopy was done the same day,42 capsule endoscopy detected additional significant findings in 36% of cases. However, performing capsule endoscopy the same day can be logistically difficult.
In patients with ulcerative colitis (Figure 1f),3 capsule endoscopy underestimates the amount of disease involvement in the large intestine, and colonoscopy is still the gold standard with its ability to obtain tissue for analysis.3
Recommendations
Capsule endoscopy should not be used to screen for or diagnose colon cancer in the general population, and especially not in those with a family history of colon cancer or alarm symptoms of anemia, bleeding, or weight loss, in whom the risk of malignancy is 5 to 10 times greater (Table 4).6
PATIENT PREPARATION FOR CAPSULE ENDOSCOPY
Iron supplements that can discolor the mucosa should be stopped at least 7 days before the procedure, and patients should generally fast for 12 hours before the procedure.
Capsule endoscopy can be done with or without laxatives for small-bowel studies, but drinking 1 gallon (4 L) of a polyethylene glycol solution (eg, Miralax) appears to allow for higher-quality images. Other strategies to improve image quality include simethicone to reduce bubbles and N-acetylcysteine to break up mucus. For large-bowel studies, as mentioned above, the cleansing preparations must be close to perfect, since the capsule has no suction or flushing capabilities.
Routine laboratory testing and radiography are not needed.
Informed consent must be obtained and should detail the benefits of the procedure and possible complications (see below).
Sensor arrays are contained in a belt worn by the patient or are placed as 8 leads similar to electrocardiographic leads, attached by adhesive pads to the abdomen and connected to a recorder worn on a belt.
Removing the capsule from its holder activates it, and it is then swallowed with water. In patients who have difficulty swallowing or had previously retained capsules in the stomach, the capsule can be deployed directly into the small intestine using an upper-GI endoscope.
Patients can start to take clear liquids 2 hours after swallowing the capsule, and medications after 4 hours. They can go about their activities of daily living, and the belt and recording devices are removed after 8 hours to be reviewed.
COMPLICATIONS OF CAPSULE ENDOSCOPY
Capsule endoscopy is relatively safe, with rare complications.
Capsule retention, ie, the capsule getting stuck somewhere along the GI tract, is the most common complication and occurs in about 2% of all studies.43 Normally, the capsule is expelled within 3 to 7 days, and therefore capsule retention is defined as occurring when the capsule remains inside for a minimum of 2 weeks.
Most cases of retention are asymptomatic. Risk factors include small-bowel obstruction, strictures, history of small-bowel surgery, abdominal or pelvic radiation therapy, and inflammatory bowel disease (Table 5).
Risk factors for capsule retention
Since radiography and magnetic resonance imaging cannot be relied on to detect significant obstructions, Medtronic has developed a dissolvable “patency capsule” to be given to patients before they undergo capsule endoscopy, to try to identify any areas where the video capsule could get stuck. The patency capsule contains a radiofrequency identifier chip covered with cellophane-filled barium and lactose, with time-released biodegradable plugs at both ends that fully dissolve in 40 to 80 hours if the capsule gets stuck in the GI tract. Passage of the retention capsule by 30 hours suggests no obstruction to impede the use of capsule endoscopy.
In a study of 106 patients,44 all patients who successfully excreted the patency capsule underwent successful capsule endoscopy, and none retained the video capsule.44
In treating capsule retention, watchful waiting is suggested, and various radiographic studies may help locate the retained capsule. In patients with inflammatory bowel disease, the use of steroids has facilitated the passage of retained capsules.45 However, leaving the capsule in for a long time may expose the patient to complications of obstruction, perforation, and capsule fragmentation, and shared decision-making with the patient may be necessary for retrieval after 2 weeks even though passage of an intact capsule has been reported after 4.5 years.46
Retained capsules can be retrieved surgically, but this approach is being marginalized by device-assisted enteroscopy, which has proven to be 90% to 100% effective in most cases.47
Other complications include battery failure, missed lesions, bowel obstruction, bowel perforation, and aspiration of the capsule into the trachea and bronchial tree.48
COST-EFFECTIVENESS
The cost-effectiveness of capsule endoscopy is difficult to ascertain and depends on various factors such as region of the country, type of procedure, inpatient vs outpatient setting, insurance coverage, facility fees, physician fees,49 and effect on length of stay.
Nevertheless, capsule endoscopy appears to be most cost-effective if performed for a disease-specific indication. For example, in surveillance of Crohn disease, capsule endoscopy proved to be cost-effective in a study by Lobo et al.50 In another study,51 the authors projected that early use of capsule endoscopy to assess upper-GI bleeding without hematemesis could lead to earlier hospital discharge, at 0.88 days compared with 1.63 days with standard care.
FUTURE CAPSULES WILL DO MORE
Passage of the video capsule depends on the unpredictable and unreliable peristaltic contractility of the GI tract, and so the inability to control and guide the movement of the capsule presents a major challenge. Research is under way to give future capsules the ability to stop, inspect, biopsy, review, tamponade, coagulate, and provide treatment with drug-delivery systems. Devices under study include MACE (Magnetic Activated Soft Capsule Endoscope), NEMO (Nano-based Capsule Endoscopy), and VECTOR (Versatile Endoscopy Capsule for Gastrointestinal Tumor Recognition and Therapy).52
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.