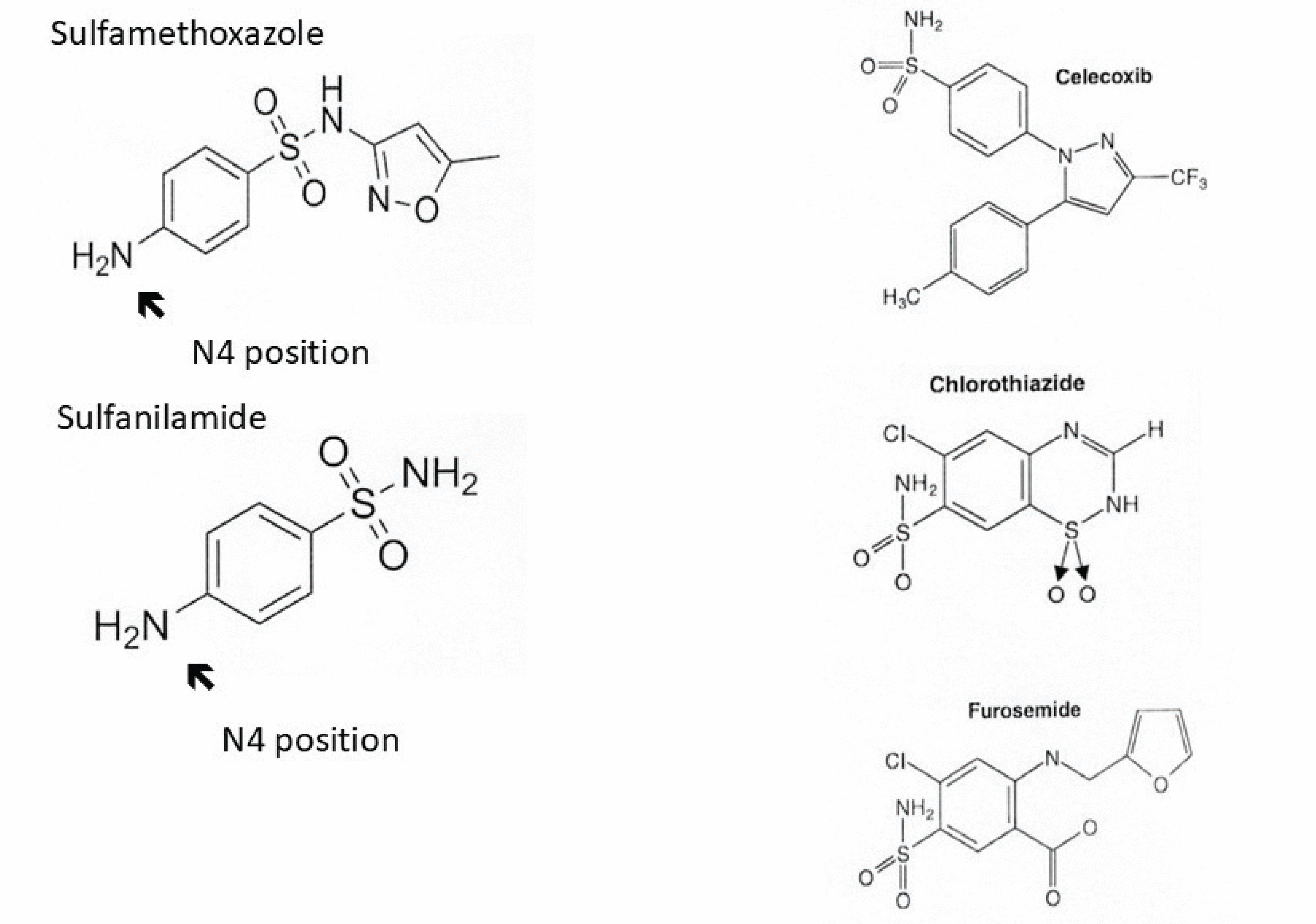
There is no cross-reactivity between antimicrobial sulfonamides and nonantimicrobial sulfonamides. For this reason, patients with a history of immunoglobulin (Ig) E–mediated (allergic or anaphylactic) reaction to a sulfonamide antibiotic can receive nonantimicrobial sulfonamides such as celecoxib, chlorthiazide, furosemide, and others without elevated risk of an IgE-mediated reaction compared with the general population.
SULFONAMIDE ALLERGY
Patients with a reported sulfonamide allergy are frequently encountered in clinical practice. A history of “sulfa allergy” is second in frequency to penicillin allergy and is reported in 3% to 6% of the general population.1–4 Clarification of allergy status is particularly important because sulfonamide antibiotics remain first-line treatments for certain infections, including Pneumocystis jirovecii, Toxoplasma gondii, and Stenotrophomonas maltophilia.5
Adverse reactions to sulfonamides vary from mild and self-limited to potentially life-threatening, and may include any of the 4 hypersensitivity reactions from the Gell and Coombs classification (Table 1).1,2 Cutaneous reactions are the most frequent, with maculopapular exanthemas being the most common type.6 Cutaneous reactions to sulfonamides have also been reported in up to 30% of patients with human immunodeficiency virus.1 Drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome, toxic epidermal necrolysis, and other severe adverse reactions are fortunately less common.5
Gell and Coombs classification of hypersensitivity reactions
CROSS-REACTIVITY BETWEEN SULFONAMIDES
Table 2 lists commonly prescribed antimicrobial and nonantimicrobial sulfonamides. These drugs all contain an SO2NH2 moiety (Figure 1), from which they derive the designation sulfonamides. Antimicrobial sulfonamides contain an arylamine group at the N4 position, which accounts for the drugs’ antimicrobial function through competitive inhibition of a structurally similar compound needed for microbial processes. This arylamine group and another nitrogen-containing ring found in antimicrobial sulfonamides are the primary targets, or determinants, for allergic sensitization.1,2
Commonly prescribed antimicrobial and nonantimicrobial sulfonamides
Chemical structures of antimicrobial and nonantimicrobial sulfonamides. Interclass reactivity and nonreactivity between antimicrobial and nonantimicrobial sulfonamides are shown. All sulfonamides contain an SO2NH2 an arylamine group at the N4 position (arrow), which serves as the primary target for immunoglobulin E–moiety. Antimicrobial sulfonamides (eg, sulfamethoxazole and sulfanilamide) contain mediated sensitization. Nonantimicrobial sulfonamides (eg, celecoxib, chlorothiazide, and furosemide) lack the arylamine group at the N4 position. For this reason, these drugs do not cross-react with antimicrobial sulfonamides.
Type I (immediate) hypersensitivity reactions occur when IgE binds and cross-links to a specific antigenic determinant. This results in the activation of mast cells and the release of inflammatory mediators, including histamine, leukotrienes, and others, which can manifest as pruritus, urticaria, angioedema, bronchospasm, vomiting, and hypotension. Thus, molecular structure determines IgE-mediated allergenicity and cross-reactivity. An index reaction to 1 antimicrobial sulfonamide agent precludes future use of other antimicrobial sulfonamides due to interclass cross-reactivity of the shared arylamine group’s allergenic determinant.7 A preferred label for this allergy would be sulfonamide antibiotics, to indicate that an alternative nonsulfonamide antibiotic should be used. Notably, these type I allergic reactions to sulfonamides are not directed at the SO2NH2 group after which the drug class is named.2
Nonantimicrobial sulfonamides include carbonic anhydrase inhibitors, selective cyclooxygenase-2 inhibitors, loop diuretics, sulfonylureas, thiazide diuretics, triptans, and other agents.2 Although product information approved by the US Food and Drug Administration for nonantimicrobial sulfonamides may include warnings about possible cross-reaction with antimicrobial sulfonamides,1 these drugs do not need to be withheld. Nonantimicrobial sulfonamides lack an arylamine group at the N4 position, so they do not cross-react with antimicrobial sulfonamides. For example, a patient with a history consistent with IgE-mediated reaction to the antimicrobial sulfonamide trimethoprim-sulfamethoxazole can receive celecoxib, chlorthiazide, furosemide, or other nonantimicrobial sulfonamides, as indicated.
Antimicrobial sulfonamide metabolites are most likely responsible for non–IgE-mediated reactions such as Stevens-Johnson syndrome or toxic epidermal necrolysis.1,2,6 Because nonantimicrobial sulfonamides lack an arylamine group, they do not produce similar metabolites, which is the reason they do not cross-react in patients who have had non–IgE-mediated reactions to antimicrobial sulfonamides.
Patients who have had IgE-mediated or non–IgE-mediated reactions to antimicrobial sulfonamides may also receive medications or other agents that contain sulfates or sulfites, such as morphine sulfate, ferrous sulfate, potassium metabisulfite, and sodium bisulfite, as these are not sulfonamides. The same recommendation applies for dapsone, a sulfone, which also does not need to be withheld.
EVALUATION AND MANAGEMENT OF SULFONAMIDE ALLERGY
Sulfonamide allergy management depends on the type of reaction and the underlying immune mechanism. Patients who report a severe delayed immune-mediated reaction (eg, drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome or toxic epidermal necrolysis, acute generalized exanthematous pustulosis, drug-induced nephritis or hepatitis) should subsequently avoid the culprit drug, as this can be regarded as a contraindication to future use.4,8–11 However, patients with more benign reactions or a suspected IgE-mediated allergy may be candidates for reevaluation.
Lack of validated testing
Clinical history combined with immediate hypersensitivity skin or in vitro testing can be used to confirm or rule out IgE-mediated allergic potential to penicillin; in vitro testing is generally not recommended based on poor sensitivity.4 In contrast to penicillin, neither skin nor in vitro testing for sulfonamide allergy has been validated.4,8 The reference standard to establish allergic potential vs tolerance is drug provocation, or direct oral challenge (DOC) with a test dose of the culprit drug.
In the absence of validated diagnostic testing, counseling for a reported “sulfa allergy” historically led to a recommendation of future sulfonamide drug avoidance. When a sulfonamide drug was clearly indicated, without an equally efficacious antibiotic that could be used, desensitization was performed to induce temporary tolerance.4 This enabled administration of a sulfonamide antibiotic to treat an acute infection, but it did not clarify whether an allergic or anaphylactic potential was present. Although effective, these protocols were lengthy, costly, and at times impractical—especially for patients needing intermittent therapy, as serial desensitization was required for temporary tolerance for each antibiotic course.8
DOC for low-risk patients
Fortunately, guidance on the approach to sulfonamide allergy has evolved to reflect more recent data showing the safety and efficacy of performing DOC in properly selected low-risk patients. A simplified algorithm for reassessment, as opposed to avoidance or desensitization, which implies a presumption of lifelong IgE-mediated potential, enables allergy “delabeling” based on history-guided DOC as standard of care.
We have learned that rates of true or persistent type I hypersensitivity to sulfonamide antibiotics are lower than previously thought.4 Accordingly, the 2022 Drug Allergy Practice Parameter update4 recommends a 1-step DOC to trimethoprim-sulfamethoxazole for low-risk patients, defined as those with a history of benign cutaneous reaction (eg, morbilliform or urticarial rash), unknown or remote history, or nonsevere delayed (> 36 hours) reaction to a sulfonamide antibiotic. As an added precaution for patients with a reaction history within the previous 5 years, which makes them higher risk, a 2-step DOC, starting with 10% of the target dose, is recommended.
This protocol is an extension of the widely accepted PEN-FAST (penicillin allergy reported by patient, five years or less since reaction, anaphylaxis or angioedema, severe cutaneous adverse reaction, and treatment required for reaction) clinical decision tool that has been used to identify patients with reported penicillin allergy who are appropriate for DOC rather than immediate hypersensitivity skin testing, which recent data suggest has poor positive predictive value in low-risk patients.11 Preliminary data for the SULF-FAST clinical decision tool have been promising, with high specificity and negative predictive value; however, further validation is required before it is implemented more widely.12
Delabeling patients
Earlier studies were directed at delabeling patients with greater need for sulfonamide antibiotics, such as trimethoprim-sulfamethoxazole for P jirovecii prophylaxis in immunosuppressed populations, including patients with cancer, human immunodeficiency virus, or acquired immunodeficiency syndrome in whom the benefit of DOC outweighed the risk.13 More recent data have shown similar levels of DOC safety and tolerance in the general population.8,10,12 Proactive delabeling for “sulfa allergy” is not yet the standard of care as it is for penicillin. However, when there is an explicit need for sulfonamide antibiotic therapy, including anticipated immunosuppression due to a future organ transplant,10 delabeling via DOC can be performed for both immunocompromised and immunocompetent patients categorized as low risk.4
THE BOTTOM LINE
Sulfonamide allergy is commonly encountered and is clinically important. Patients with a history of severe cutaneous or other serious delayed-type reaction (eg, drug reaction with eosinophilia and systemic symptoms or Stevens-Johnson syndrome) to an antimicrobial sulfonamide should be cautioned to maintain lifelong avoidance. Patients with a history of recent IgE-mediated (allergic or anaphylactic) reaction should empirically avoid all antimicrobial sulfonamides based on the risk of cross-reaction; however, nonantimicrobial sulfonamides do not need to be avoided. In addition, a 1- or 2-step DOC can be considered for properly selected low-risk patients. The allergy label should accurately reflect the restrictions above rather than broadly implicating all sulfonamides, as there is no evidence of cross-reactivity between antimicrobial and nonantimicrobial sulfonamides.
DISCLOSURES
Dr. Lang has disclosed consulting for Astra Zeneca, Celldex Therapeutics, Genentech, and Novartis, and teaching and speaking for Sanofi Regeneron. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2025 The Cleveland Clinic Foundation. All Rights Reserved.