A 49-year-old man developed nephrotic-range proteinuria (urine protein–creatinine ratio 4.1 g/g), and primary membranous nephropathy was diagnosed by kidney biopsy. He declined therapy apart from angiotensin receptor blockade.
Five months after undergoing the biopsy, he presented to the emergency room with marked dyspnea, cough, and epigastric discomfort. His blood pressure was 160/100 mm Hg, heart rate 95 beats/minute, and oxygen saturation by pulse oximetry 97% at rest on ambient air, decreasing to 92% with ambulation.
Initial laboratory testing results were as follows:
Sodium 135 mmol/L (reference range 136– 144)
Potassium 3.9 mmol/L (3.7–5.1)
Chloride 104 mmol/L (97–105)
Bicarbonate 21 mmol/L (22–30)
Blood urea nitrogen 14 mg/dL (9–24)
Serum creatinine 1.1 mg/dL (0.73–1.22)
Albumin 2.1 g/dL (3.4–4.9).
Urinalysis revealed the following:
5 red blood cells per high-power field, compared with 1 to 2 previously
3+ proteinuria
Urine protein–creatinine ratio 11 g/g
No glucosuria.
Electrocardiography revealed normal sinus rhythm without ischemic changes. Chest radiography did not show consolidation.
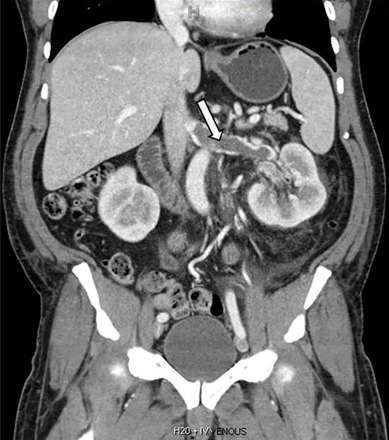
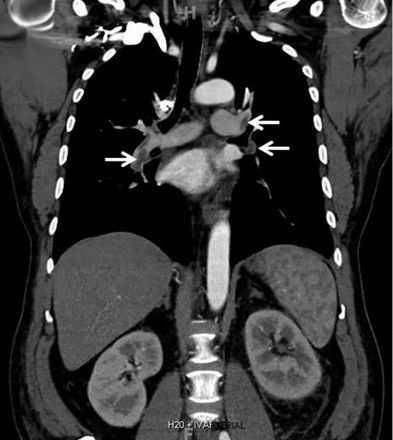
Computed tomography of the chest and abdomen with intravenous contrast demonstrated a nearly occlusive thrombus in the left renal vein (Figure 1) extending to the inferior vena cava with bilateral, nearly occlusive pulmonary emboli (Figure 2).
Coronal reformatted contrast-enhanced computed tomography showed a nearly occlusive low-attenuation filling defect within the left renal vein (arrow).
Coronal reformatted contrast-enhanced computed tomography of the chest showed bilateral low-attenuation filling defects in the pulmonary arteries (arrows).
The patient was started on systemic anticoagulation with unfractionated heparin, which was then transitioned to warfarin therapy. Immunosuppressive therapy was also started, with rituximab 1,000 mg every other week for 2 doses, and 6 months of alternating monthly oral therapy with cyclophosphamide and methylprednisolone.
At 7 months after the thrombotic event, there was no evidence of residual renal vein thrombosis on magnetic resonance venography, and at 14 months his serum creatinine level was 0.9 mg/dL, albumin 4.0 g/dL, and urine protein–creatinine ratio 0.8 g/g.
RENAL VEIN THROMBOSIS: RISK FACTORS AND CLINICAL FEATURES
Severe hypoalbuminemia in the setting of nephrotic syndrome due to membranous nephropathy is associated with the highest risk of venous thromboembolic events, with renal vein thrombus being the classic complication.1 Venous thromboembolic events also occur in other nephrotic syndromes, albeit at a lower frequency.2
Venous thromboembolic events are estimated to occur in 7% to 33% of patients with membranous glomerulopathy, with albumin levels less than 2.8 g/dL considered a notable risk factor.1,2
While often a chronic complication, acute renal vein thrombosis may present with flank pain and hematuria.3 In our patient, the dramatic increase in proteinuria and possibly the increase in hematuria suggested renal vein thrombosis. Proximal tubular dysfunction, such as glucosuria, can be seen on occasion.
DIAGNOSIS AND TREATMENT
Screening asymptomatic patients for renal vein thrombosis is not recommended, and the decision to start prophylactic anticoagulation must be individualized.4
Although renal venography historically was the gold standard test to diagnose renal vein thrombosis, it has been replaced by noninvasive imaging such as computed tomography and magnetic resonance venography.
While anticoagulation remains the treatment of choice, catheter-directed thrombectomy or surgical thrombectomy can be considered for some patients with acute renal vein thrombosis.5
- Copyright © 2018 The Cleveland Clinic Foundation. All Rights Reserved.