ABSTRACT
Over the last 50 years, the use of transvenous pacemakers has been constrained by long-term complications that affect more than 1 in 10 patients, largely attributable to the endovascular leads and surgical pocket. Leadless cardiac pacing involves a self-contained pacemaker deployed directly into the heart without a lead or incisional access. The procedure has shown promise, eliminating pocket-related complications. Other advantages include postprocedural shoulder mobility and the ability to drive, shower, and bathe. Current devices are limited to single-chamber ventricular pacing. Future advances may allow atrial and dual-chamber pacing and combination with a subcutaneous defibrillator to deliver antitachycardia pacing and provide bradycardia backup.
Leadless cardiac pacing has emerged as a safe and effective alternative involving catheter-based delivery of a self-contained device directly into the right ventricle without incisional access, leads, or a surgical pocket. The procedure typically can be performed in 30 minutes or less, with fewer postprocedure restrictions.
Leadless pacing is showing promising results, but it is currently limited to single-chamber pacing.
Future directions include atrial and dual-chamber pacing and combining the procedure with a subcutaneous implantable cardioverter-defibrillator.
WHY LEADLESS PACING?
The first clinical implantation of a cardiac pacemaker was performed surgically in 1958 by Drs. Elmvist and Senning via thoracotomy and direct attachment of electrodes to the myocardium. Transvenous pacing was introduced in 1962 by Drs. Lagergren, Parsonnet, and Welti.1,2 The general configuration of trans-venous leads connected to a pulse generator situated in a surgical pocket has remained the standard of care ever since. Despite almost 60 years of technological innovation, contemporary permanent transvenous pacing continues to carry significant short- and long-term morbidity. Long-term composite complication rates are estimated at over 10%,3 further stratified as 12% in the 2 months post-implant (short-term) and 9% thereafter (long-term).4 Transvenous pacing complications are associated with an increase in both hospitalization days (hazard ratio 2.3) and unique hospitalizations (hazard ratio 4.4).5
Short-term complications
Short-term complications include lead dislodgment, pocket hematoma, pericardial effusion, and pneumo-thorax (Figure 1). Pocket hematomas are common with concurrent antiplatelet or anticoagulant administration, with incidence estimates varying from 5% to 33% depending on the definition.6 Morbidity associated with pocket hematoma include prolonged hospitalization, need for re-operation,7 and an almost eightfold increase in the rate of device infection over the long term compared with patients without pocket hematoma.8 New pericardial effusions after implant may affect up to 10% of patients; they are generally small, including 90% attributable to pericarditis or contained microperforation not requiring intervention. Overt lead perforation resulting in cardiac tamponade occurs in about 1% of transvenous pacemaker implants, of which 10% (0.1% overall) require open chest surgery, with the remainder treated with percutaneous drainage.9
Common transvenous pacemaker lead and pocket-related complications.
Source: Lead fracture and pocket infection images courtesy of Dr. Mohamed Kanj. Hematoma image courtesy of Dr. John Rickard.
Long-term complications
Long-term complications are predominantly lead and pocket-related but also include venous occlusive disease and tricuspid valve pathology.4 The development of primary lead failure due to insulation defects, conductor fracture, or dislodgment has been associated with major adverse events in 16% of patients, and an additional 6% if transvenous lead extraction is needed, which can rarely lead to hemorrhagic death by vascular tears involving the heart or superior vena cava.10 Fibrous tissue growth around the indwelling vascular leads can result in venous obstruction present in up to 14% of patients by 6 months after implant.11 This increases to 26% by the time of device replacement or upgrade, which is typically 5 to 10 years after the original implant, including 17% of patients with a complete venous occlusion.12 In addition, worsened tricuspid regurgitation due to lead impingement on the valve is seen in 7% to 40% of patients depending on definitions,13 with post-implant severe tricuspid regurgitation independently associated with increased mortality risk.14 The rate of device infection is 1% to 2% at 1 year,8,15 and 3% over the lifetime of the initial transvenous system; this increases to more than 10% after generator replacement.16
LEADLESS PACING TECHNOLOGY
The principal goal of leadless pacing is to reduce short- and long-term pacemaker complications by eliminating the two most common sources of problems: the transvenous leads and the surgical pocket. Discussion of leadless pacing strategies began as early as 1970.17 Although several preclinical studies demonstrated efficacy with leadless prototypes,18–20 clinical implementation of fully leadless technology did not occur until recently. As shown in Figure 2, there are now two commercially available leadless pacing devices: Nanostim (St. Jude Medical Inc., St. Paul, MN) and Micra (Medtronic Inc., Dublin, Ireland). At the time of this writing, both have commercial approval in Europe. In the United States, Micra received commercial approval from the US Food and Drug Administration on April 6, 2016, with a similar decision expected on Nanostim. The current approved indications for leadless pacing are chronic atrial tachyarrhythmia with advanced atrioventricu-lar (AV) block; advanced AV block with low level of physical activity or short expected lifespan; and infrequent pauses or unexplained syncope with abnormal findings at electrophysiologic study. Although differ ences exist between Nanostim and Micra, as shown in Table 1,21–27 there are fundamental similarities. Both are single-unit designs encapsulating the electrodes and pulse generator with rate-adaptive functionality. Both are delivered via an endovascular femoral venous approach without the need for incisional access, trans-venous leads, or surgical pocket (Figures 3 and 4).21–27
Leadless pacemakers (A) Nanostim and (B) Micra.
Overview of leadless pacemakers Nanostim and Micra based on completed human trials
Fluoroscopic images depicting catheter-based deployment and subsequent release for the (A) Nanostim and (B) Micra.
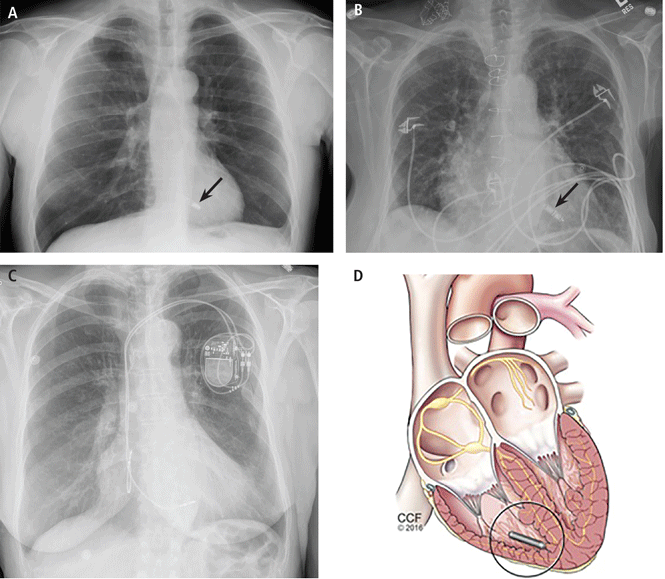
Frontal-plane radiographs showing implanted Nanostim (A) and Micra (B) leadless pacing devices and a traditional dual-chamber pacemaker (C). Panel D depicts cardiac deployment.
Nanostim: Landmark trials
As the world’s first-in-man leadless pacemaker, Nanostim was evaluated in two prospective, non-randomized, multicenter, single-arm trials abbreviated LEADLESS22 and LEADLESS II.24 The first human feasibility study, LEADLESS, enrolled 33 patients with approved indications for ventricular-only pacing while excluding patients with expected pacemaker dependency. The most common indication was bradycardia in the presence of persistent atrial arrhythmias, thereby obviating the need for atrial pacing. The primary outcome was freedom from serious complications at 90 days. The secondary outcomes were implant success rate and device performance at 3 months. The results demonstrated 94% composite safety (31 of 33 patients) at 3 months. There was one cardiac perforation leading to tampon-ade and eventually death after prolonged hospitalization, and one inadvertent deployment into the left ventricle via patent foramen ovale that was successfully retrieved and redeployed without complication. The implant success rate was 97%, and the electrical parameters involving sensing, pacing thresholds, and impedance were as expected at 3 months. Results of 1-year follow-up were published for the LEADLESS cohort,25 revealing no additional complications from 3 to 12 months, no adverse changes in electrical performance parameters, and 100% effectiveness of rate-responsive programming.
The subsequent LEADLESS II trial enrolled 526 patients but did not exclude patients with expected pacemaker dependency, and its results were reported in a preplanned interim analysis when 300 patients had reached 6 months of follow-up (mean follow-up 6.9 ± 4.2 months).24 The primary efficacy end point involved electrical performance including capture thresholds and sensing. Initial deployment success was 96% with expected electrical parameters at implant that were stable at 6 months of follow-up. The rate of freedom from serious adverse events was 93%, with complications including device dislodgment (1.7%, mean 8 ± 6 days after implant), perforation (1.3%), performance deficiency requiring device retrieval and replacement (1.3%), and groin complications (1.3%). There were no device-related deaths, and all device dislodgments were successfully treated percutaneously.
There was no prospective control arm involving transvenous pacing in either the LEADLESS or LEADLESS II trial. Thus, in an effort to compare Nanostim (n = 718) vs transvenous pacing, complication rates were calculated for a propensity-matched registry cohort of 10,521 transvenous patients, and differences were reported.26 At 1 month, the composite complication rate was 5.8% for Nanostim (1.5% pericardial effusion, 1% dislodgment) and 12.7% for transvenous pacing (7.6% lead-related, 3.9% thoracic trauma, infection 1.9%) (P < .001). Between 1 month and 2 years, complication rates were only 0.6% for Nanostim vs 5.4% for transvenous pacing (P < .001). This lower complication rate at 2 years was driven almost entirely by a 2.6% infection rate and 2.4% lead-complication rate in the transvenous pacemaker group, nonexistent in the leadless group.
Micra: Landmark trials
Micra was evaluated in a prospective, nonrandom-ized, multicenter, single-arm trial, enrolling 725 patients with indications for ventricular-only pacing; approximately two-thirds of the cohort had bradycar-dia in the presence of persistent atrial arrhythmias, similar to the Nanostim cohort.27 The efficacy end point was stable capture threshold at 6 months. The safety end point was freedom from major complications resulting in new or prolonged hospitalization at 6 months. The implant success rate was 99%, and 98% of patients met the primary efficacy end point.
The safety end point was met in 96% of patients. Complications included perforation or pericardial effusion (1.6%), groin complication (0.7%), elevated threshold (0.3%), venous thromboembolism (0.3%), and others (1.7%). No dislodgments were reported. There was no prospective, randomized control arm to compare Micra and transvenous pacing. A post hoc analysis was performed comparing major complication rates in this study with an unmatched 2,667-patient meta-analysis control cohort.27 The hazard ratio for the leadless pacing strategy was calculated at 0.49 (95% confidence interval 0.33 to 0.75, P = .001) with absolute risk reduction 3.4% at 6 months resulting in a number needed to treat of 29.4 patients. Further broken down, Micra patients compared with the control cohort had reduced rates of both subsequent hospitalizations (3.9% to 2.3%) and device revisions (3.5% to 0.4%).
ADVANTAGES OF LEADLESS PACING
As discussed above, the major observed benefit with both Nanostim and Micra compared with trans-venous cohorts is the elimination of lead and pocket-related complications.25,27 Leadless pacing introduces a new 1% to 2% groin complication rate for both devices not present with transvenous pacing, and also a 1% device dislodgment rate in the case of Nanostim (all dislodgments were treated per-cutaneously). Data from both clinical trials suggest that the complication rates are largely compressed acutely. In contrast, there are considerable midterm and long-term complications for trans venous systems.3–5 Indeed, the mid- to long-term window is where leadless pacing is expected to have the most favorable impact. As with any new disruptive technology, operator experience may be important, as evidenced by a near halving of the complication rate observed in the LEADLESS II trial after gaining the experience of 10 implants.25
Other benefits of leadless pacing include a generally quick procedure (average implant time was 30 minutes in LEADLESS and LEADLESS II)22,25 and full shoulder mobility afterwards, so that patients can resume driving once groin soreness has subsided, typically within a few days. (Current studies are investigating whether immediate shoulder mobility with leadless pacing is beneficial to older patients suffering from arthritis.) The lack of an incision allows patients to bathe and shower as soon as they desire, whereas after transvenous pacemaker implant, motion in the affected shoulder is usually restricted for several weeks to avoid lead dislodgment, and showering and bathing are restricted to avoid contamination of the incision with nonsterile tap water. (In some cases, a tightly adherent waterproof dressing can be used.) The leadless systems were designed for compatibility with magnetic resonance imaging (MRI), whereas not all transvenous pacemaker generators and leads are MRI compatible.
Leadless devices are not expected to span the tri-cuspid valve to create incident or worsening tricuspid regurgitation. In a recent small study of 22 patients undergoing Micra implant, there were no new cases of severe tricuspid regurgitation after the procedure, with only a 9% increase in mild and 5% increase in moderate tricuspid regurgitation,28 vs a rate of 40% of worsening tricuspid regurgitation and 10% of new severe tricuspid regurgitation with transvenous pacing.13,14
Transvenous pacemaker implant requires surgery for pulse generator exchange at a mean of 7 years, a procedure carrying significant risk of short- and long-term complications.10
END-OF-SERVICE QUESTIONS: ATTEMPT RETRIEVAL OR NOT?
Both leadless systems have favorable projected in-service battery life: a reported 15.0 years for Nanostim25 and mean 12.5 years for Micra.27 The inevitable question is what to do then. The Nanostim system was designed to be retrievable using a dedicated catheter system. Micra was not designed with an accompanying retrieval system. Pathologic examinations of leadless devices at autopsy or after explant have revealed a range of device endothelialization, from partial at 19 months to full at 4 months.29,30
As of this writing, no extraction complications have been observed with Nanostim explants up to 506 days after implant (n = 12, mean 197 days after implant).31 Needless to say, there is not yet enough experience worldwide with either system to know what the end-of-service will look like in 10 to 15 years. One strategy could involve first attempting per-cutaneous retrieval and replacement, if retrieval is not possible, abandoning the old device while implanting a new device alongside. Another strategy would be to forgo a retrieval attempt altogether. In the LEADLESS II study,24 the mean patient age was 75. In this cohort, forgoing elective retrieval for those who live to reach the end of pacemaker service between the age of 85 and 90 would seem reasonable assuming the next device provides similar longevity. For younger patients, careful consideration of long-term strategies is needed. It is not known what the replacement technology will look like in another decade with respect to device size or battery longevity. Preclinical studies using swine and human cadaver hearts have demonstrated the feasibility of multiple right-ventricular Micra implants without affecting cardiac function.32,33
OTHER LIMITATIONS AND CAUTIONARY NOTES
At present, leadless pacing is approved for single-chamber right-ventricular pacing. In the developed world, single right-ventricular pacing modes account for only 20% to 30% of new pacemaker implants, which total more than 1 million per year worldwide.34,35 As with any new technology, the up-front cost of leadless pacemaker implant is expected to be significantly higher than transvenous systems, which at this point remains poorly defined, as the field has not caught up in terms of charges, reimbursement, and billing codes. While those concerns fall outside the scope of this review, it is not known if the expected reductions in mid- and long-term complications will make up for an up-front cost difference. However, a cost-efficacy study reported that one complication of a transvenous pacemaker system was more expensive than the initial implant itself.36 The longest-term follow-up data currently available are with Nano stim, showing an absolute complication reduction of 11.7% at 2 years,24 a disparity only expected to widen with prolonged follow-up, particularly after transvenous generator exchange, when complication rates rapidly escalate.
FUTURE DIRECTIONS
The next horizon of leadless technology will be for right-atrial and dual-chamber pacing to treat the far more pervasive pacing indication of sinus node dysfunction with or without AV block. In the latter application, the two devices will communicate. Proto types and early nonhuman evaluations are ongoing for both. Leadless pacing is also being investigated for use in tachycardia. Tjong et al37 reported on the safety and feasibility of an entirely leadless pacemaker plus an implantable cardioverter-defi-brillator (ICD) system in two sheep and one human using both Nanostim and subcutaneous ICD. Currently, two important limitations of subcutaneous ICD are its inability to provide backup bradycardia and antitachycardia pacing (it provides only defibril-lation). The EMBLEM PACE study will enroll 250 patients to receive a leadless pacemaker and Emblem subcutaneous ICD (Boston Scientific, Boston, MA), with patients subsequently receiving commanded antitachycardia pacing for ventricular arrhythmias and bradycardia pacing provided by the leadless device as indicated.
CONCLUSIONS
Leadless cardiac pacing is a safe and efficacious alternative to standard transvenous pacing systems. Although long-term data are limited, available short- and mid-term data show that the elimination of transvenous leads and the surgical pocket results in significant reductions in complication rates. Currently, leadless pacing is approved only for right-ventricular pacing, but investigation of right-atrial, dual-chamber, and fully leadless pacemaker-defibrillator hybrid systems is ongoing.
Footnotes
Dr. Kiehl reported no financial interests or relationships that pose a potential conflict of interest with this article. Dr. Cantillon reported consulting for Boston Scientific Corporation and St. Jude Medical; membership on advisory committees for Boston Scientific Corporation and St. Jude Medical; and teaching/ speaking for Boston Scientific Corporation.
- Copyright © 2016 The Cleveland Clinic Foundation. All Rights Reserved.