ABSTRACT
Cardiopulmonary exercise testing (CPET) helps in detecting disorders of the cardiovascular, pulmonary, and skeletal muscle systems. It has a class I (indicated) recommendation from the American College of Cardiology and American Heart Association for evaluating exertional dyspnea of uncertain cause and for evaluating cardiac patients being considered for heart transplant. Advances in hardware and software and ease of use have brought its application into the clinical arena to the point that providers should become familiar with it and consider it earlier in the evaluation of their patients.
Technological advances and ease of use have brought CPET out of specialized centers and into the realm of daily clinical practice.
CPET is a versatile test that has unique ability to assess cardiopulmonary and metabolic responses to exercise that can reflect underlying pathology.
CPET has established value in assessing patients with exertional dyspnea and can guide clinical decision-making and help streamline patient management by focusing on the cause or excluding pathology.
CPET has useful prognostic capabilities in patients with heart failure to guide medical treatment or referral for advanced therapies.
Cardiopulmonary exercise testing (CPET) is a versatile tool that can be useful in patient management and clinical decision-making. Many physicians are unfamiliar with it, in part because historically it was cumbersome, done mostly in research or exercise physiology centers, and used mostly in assessing athletic fitness rather than pathologic conditions. In addition, medical schools provide little instruction about it, and hands-on use has typically been relegated to pulmonologists.
Improvements in hardware and software and ease of use have brought this test into the clinical arena to the point that clinicians should consider it earlier in the evaluation of appropriate patients. It now has a class I recommendation (ie, the test is indicated) from the American College of Cardiology and American Heart Association for evaluating exertional dyspnea of uncertain cause and for evaluating cardiac patients being considered for transplant.1 It also is a powerful prognosticator of outcomes in heart failure patients.
CARDIOPULMONARY EXERCISE TESTING MADE SIMPLE
CPET is the analysis of gas exchange during exercise. Modern systems measure, breath-by-breath, the volume of oxygen taken up (Vo2), and the volumes of carbon dioxide (Vco2) and air expired (VE).
Testing can be done with nearly any kind of exercise (treadmill, cycle, arm ergometry), thus accommodating patient or provider preference. Most exercise protocols involve a gradual increase in work rather than increasing stages of work for smooth data collection, and graphical display for optimal test interpretation.
After undergoing baseline screening spirometry, the patient rides a stationary bicycle or walks on a treadmill while breathing through a nonrebreathing mask and wearing electrocardiographic leads, a blood pressure cuff, and a pulse oximeter. The test starts out easy and gets progressively harder until the patient fatigues, reaches his or her predicted peak Vo2, or, as in any stress test, experiences any other clinical indication for stopping, such as arrhythmias, hypotension, or symptoms (rare). We advise patients to wear comfortable workout clothes, and we ask them to try as hard as they can. The test takes about 10 to 15 minutes. Patients are instructed to take all of their usual medications, including beta-blockers, unless advised otherwise at the discretion of the supervising physician.
What the numbers mean
Table 1 lists common CPET variables; Table 2 lists common patterns of results and what they suggest. Other reviews further discuss disease-specific CPET patterns.2–5
Selected cardiopulmonary exercise testing variables
What cardiopulmonary exercise test patterns suggest
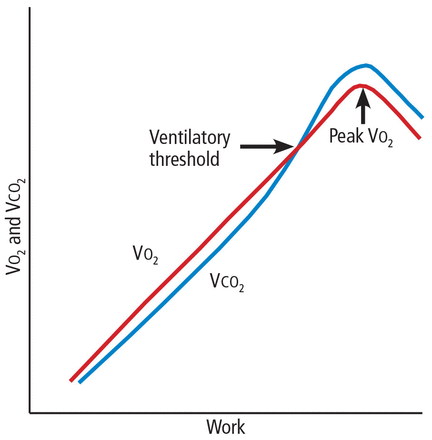
Peak Vo2. As the level of work increases, the body needs more oxygen, and oxygen consumption (Vo2) increases in a linear fashion up to a peak value (Figure 1). Peak Vo2 is the central variable in CPET. Whereas elite athletes have high peak Vo2 values, patients with exercise impairment from any cause have lower values, and average adults typically have results in the middle. Peak Vo2 can be expressed in absolute terms as liters of oxygen per minute, in indexed terms as milliliters of oxygen per kilogram of body weight per minute, and as a percentage of the predicted value.
Diagram of response to work. Impairment from any cause will lower the peak Vo2 and ventilatory threshold.
Ventilatory threshold. Before people reach their peak Vo2, they reach a point where the work demand on the muscles exceeds the oxygen that is being delivered to them, and their metabolism becomes more anaerobic. This point is called the anaerobic threshold, or more precisely the ventilatory threshold. In states of deconditioning or disease, this threshold is often lower than predicted. It can be detected either directly by measuring blood lactate levels or, more often, indirectly from the Vo2, Vco2, and Ve data (Figure 2).
One method of determining the ventilatory threshold is to determine the intersection of the Ve/Vo2 and Vco2 curves.
Ve/Vco2 slope. As exercise impairment advances, ventilatory efficiency worsens. Put simply, the demands of exercise result in greater ventilatory effort at any given level of work. This is a consequence of ventilation-perfusion mismatching from a milieu of metabolic, ventilatory, and cardiac dysregulation that accompanies advanced cardiopulmonary or metabolic disease.6,7 The most validated CPET variable reflecting this is the minute ventilation-carbon dioxide relationship (Ve/ Vco2 slope) (Figure 3).
The Ve/Vco2 slope is elevated in advanced heart failure and other hemodynamically significant cardiopulmonary conditions.
Coupled with other common CPET variables and measures such as screening spirometry, electrocardiography, heart and respiratory rate responses, pulse oximetry, and blood pressure, the Ve/Vco2 allows for a detailed and integrated assessment of exercise performance.
USING CPET TO EVALUATE EXERTIONAL DYSPNEA
Shortness of breath, particularly with exertion, is a common reason patients are referred to internists, pulmonologists, and cardiologists. It is a nonspecific symptom for which a precise cause can be elusive. Possible causes range from physical deconditioning due to obesity to new or progressive cardiopulmonary or muscular disease.
If conventional initial studies such as standard exercise testing, echocardiography, or spirometry do not definitively identify the problem, CPET can help guide additional investigation or management. Any abnormal patterns seen, together with the patient’s clinical context and other test results, can give direction to additional evaluation.
Table 2 outlines various CPET patterns that can suggest clinically significant cardiac, pulmonary, or muscle disorders.8–13 Alternatively, normal responses reassure the patient and clinician, since they suggest the patient does not have clinically significant disease.
Case 1: Obesity and dyspnea
You evaluate a 53-year-old mildly obese man for dyspnea. Cardiology evaluation 1 year earlier included normal transthoracic and stress echocardiograms. He is referred for CPET.
His peak Vo2 is low in indexed terms (22.3 mL/kg/min; 74% of predicted) but 90% of predicted in absolute terms (2.8 L/min), reflecting the contribution of his obesity. His ventilatory threshold is near the lower end of normal (50% of peak Vo2), and all other findings are normal. You conclude his dyspnea is due to deconditioning and obesity.
Case 2: Diastolic dysfunction
You follow a normal-weight 65-year-old woman who has long-standing exertional dyspnea. Evaluation 1 year ago included an echocardiogram showing a normal left ventricular ejection fraction and grade II (moderate) diastolic dysfunction, a normal exercise stress test (details were not provided), normal pulmonary function testing, and high-resolution computed tomography of the chest. She too is referred for CPET.
The findings include mild sinus tachycardia at rest and low peak Vo2 (23.7 mL/kg/ min; 69% of predicted). The Ve/Vco2 slope is substantially elevated at 43. Other measures of cardiopulmonary impairment and ventilatory inefficiency such as the end-tidal Pco2 response, oxygen uptake efficiency slope, and oxygen-pulse relationship (O2-pulse, a surrogate for stroke volume) are also abnormal. In clinical context this suggests diastolic dysfunction or unappreciated pulmonary hypertension. You refer her for right heart catheterization, which confirms findings consistent with diastolic dysfunction.
Case 3: Systemic sclerosis
A 64-year-old woman with systemic sclerosis, hypertension, diabetes, and sleep apnea is referred for CPET evaluation of dyspnea. Echocardiography 6 months ago showed a normal left ventricular ejection fraction and moderate diastolic dysfunction.
She undergoes screening spirometry. Results are abnormal and suggest restrictive disease, borderline-low breathing reserve, and low peak Vo2 (20 mL/kg/min; 71% of predicted). She also has chronotropic incompetence (peak heart rate 105 beats per minute; 67% of predicted). These findings are thought to be manifestations of her systemic sclerosis. You refer her for both pulmonary and electrophysiology consultation.
Case 4: Mitral valve prolapse
A generally healthy 73-year-old woman undergoes echocardiography because of a murmur. Findings reveal mitral valve prolapse and mitral regurgitation, which is difficult to quantify. She is referred for CPET as a noninvasive means of assessing the hemodynamic significance of her mitral regurgitation.
Her overall peak Vo2 is low (15 mL/kg/ min). The Ve/Vco2 slope is elevated at 32 (normal < 30), and end-tidal Pco2 response is also abnormal. The recovery heart rate is also abnormally elevated. Collectively, these findings indicate that her mitral valve regurgitation is hemodynamically significant, and you refer her for mitral valve surgery.
CPET’S ROLE IN HEART FAILURE
Over 2 decades ago, the direct measure of peak Vo2 during exercise was found to be an important prognosticator for patients with advanced heart failure and thus became a conventional measure for stratifying patients most in need of a heart transplant.14 To this day, a peak Vo2 of 14 mL/kg/min remains a prognostic threshold—values this low or less carry a poor prognosis.
Additional CPET variables are prognostically useful, both independently and with each other. Many of them reflect the ventilatory and metabolic inefficiencies that result from the extensive central and peripheral pathophysiology seen in heart failure.7,15–17
An elevated Ve/Vco2 slope is a strong predictor of adverse outcomes for patients with heart failure with either reduced or preserved ejection fraction.18,19 Other recognized prognostic indicators include20–23:
Low end-tidal Pco2
Exercise oscillatory breathing
Low oxygen uptake efficiency slope. All of these are readily provided in the reports of modern CPET systems. Explanations are in Table 1.
Collectively, these variables are strong predictors of outcomes in heart failure patients in terms of survival, adverse cardiac events, or progression to advanced therapy such as a left ventricular assist device or transplant. A multi-center consortium analyzed CPET results from more than 2,600 systolic heart failure patients and devised a scoring system for predicting outcomes (Table 3). This scoring system is a recommended component of the standard evaluation in patients with advanced heart failure.24
Cardiopulmonary exercise testing scoring system for patients with heart failure
EXERCISE TEST REPORTING
Currently there is no universal reporting format for CPET. Using a systematic approach such as the one proposed by Guazzi et al5 can help assure that abnormal values and patterns in all areas will be identified and incorporated in test interpretation. Table 4 lists suggested components of a CPET report and representative examples.
Suggested components of a cardiopulmonary exercise testing report
OTHER USES OF EXERCISE TESTING
CPET has also been found useful in several other clinical conditions that are beyond the scope of this review. These include pulmonary hypertension,25 differentiation of pathologic vs physiologic hypertrophy of the left ventricle,26 preclinical diastolic dysfunction,27,28 congenital heart disease in adults,29 prediction of postoperative complications in bariatric surgery,30 preoperative evaluation for lung resection and pectus excavatum,31,32 hemodynamic impact of mitral regurgitation,33 and mitochondrial myopathies.34
COST-EFFECTIVENESS UNKNOWN
The Current Procedural Terminology code for billing for CPET is 94621 (complex pulmonary stress test). The technical fee is $1,605, and the professional fee is $250. The allowable charges vary according to insurer, but under Medicare A and B, the charges are $258.93 and $70.65, respectively, of which patients typically must copay 20%. Total relative value units are 4.60, of which 1.95 are work relative value units.
The cost-effectiveness of CPET has not been studied. As illustrated in the case examples, patients often undergo numerous tests before CPET. While one might infer that CPET could streamline testing and management if done sooner in disease evaluation, this hypothesis has not been adequately studied, and further research is needed to determine if and how doing so will affect overall costs.
IMPLICATIONS FOR PRACTICE
Newer hardware and software have made CPET more available to practicing clinicians.
CPET has proven value in evaluating patients with exertional dyspnea. If first-line evaluation has not revealed an obvious cause of a patient’s dyspnea, CPET should be considered. This may avoid additional testing or streamline subsequent evaluation and management. CPET also has an established role in risk stratification of those with heart failure.
The clinical application of CPET continues to evolve. Future research will continue to refine its diagnostic and prognostic abilities in a variety of diseases. Most major hospitals and medical centers have CPET capabilities, and interested practitioners should seek out those experienced in test interpretation to increase personal familiarity and to foster appropriate patient referrals.
Manuscript submitted while the author was stationed at San Antonio Military Medical Center, San Antonio, TX.
- Copyright © 2017 The Cleveland Clinic Foundation. All Rights Reserved.