ABSTRACT
As the obesity epidemic worsens, more people are opting for weight-loss surgery, including gastric bypass. Of the possible complications associated with this procedure, hypoglycemia secondary to hyperinsulinemia is becoming a more common and therefore more relevant problem.
The differential diagnosis for endogenous causes of hyperinsulinemic hypoglycemia after gastric bypass surgery includes insulinoma, late dumping syndrome, and postgastric bypass hypoglycemia (PGBH).
The Whipple triad consists of measured low blood glucose, symptoms of low blood glucose, and reversal of symptoms when low blood glucose is corrected. If the triad is not present, then hypoglycemia is not causing the patient’s symptoms.
PGBH should initially be treated with a high-protein, high-fiber, low-carbohydrate diet and then, if hypoglycemia persists, by medication (initially acarbose, then a calcium channel blocker and octreotide or diazoxide or both).
PGBH ranges from mild, in which neuroglycopenia resolves with dietary changes with or without acarbose, to severe, in which neuroglycopenia persists despite dietary changes and multiple drugs.
Gastric bypass reversal and pancreatic surgery are a last resort for patients with debilitating neuroglycopenia when dietary modification and drug therapy fail.
Bariatric surgery, though beneficial, is associated with complications, one of which is post-gastric bypass hypoglycemia (PGBH).1 The mean time from gastric bypass to documented hypoglycemia is about 28 months.2
PGBH is probably more common than initially thought. In older reports, the prevalence was only 0.1% to 0.36%.1,3 In contrast, in a mail survey in 2015,4 one-third of bariatric surgery patients reported symptoms that raised the suspicion of hypoglycemia. Those with suspicious symptoms were more likely to have undergone Roux-en-Y surgery, to have had no preoperative diabetes, to have had a longer interval since surgery, and to be female. Restricting the suspicion of postprandial hypoglycemia to those who reported more serious symptoms, including needing third-party assistance, the prevalence was 11.6%.
Kefurt et al5 followed Roux-en-Y patients who wore a continuous glucose monitor for 86 months after surgery and found that 38% had hypoglycemia; however, symptoms of hypoglycemia were not discussed.
Thus, the exact prevalence is currently unknown. But as time goes by and more procedures are performed, the incidence will likely rise.
OBESITY IS ON THE RISE, AND SO IS WEIGHT-LOSS SURGERY
Obesity is rampant, and its prevalence continues to rise. In 2011-2012, more than two-thirds of adults in the United States were reported as obese.6 Complications of obesity such as cardiac disease, diabetes, and cancer lead to increased mortality risk.7 Obesity is difficult to reverse, as many people fail to lose weight with diet, exercise, and pharmacotherapy.
Given the difficulty of losing weight and the complications that arise from obesity, bariatric surgery has become increasingly popular. Not only do patients lose significantly more weight with bariatric surgery than with conventional measures, but surgery also reduces and often cures conditions associated with obesity.8
Nguyen et al9 reported that 671,959 patients underwent gastric bypass procedures in the United States from 2003 to 2008. In a registry maintained by the American Society for Metabolic and Bariatric Surgery10 from June 2007 to May 2009, the most common bariatric procedure in the United States was Roux-en-Y gastric bypass, followed by sleeve gastrectomy.
DIFFERENTIAL DIAGNOSIS AND DEFINITIONS
The differential diagnosis for hyperinsulinemic hypoglycemia after gastric bypass surgery includes exogenous and endogenous causes (Table 1). Exogenous causes include abuse of insulin secretagogues such as sulfonylureas or meglitinides and abuse of insulin, which may occur in patients with Munchausen syndrome, Munchausen syndrome by proxy, or malingering. Endogenous causes include insulinoma, early and late dumping syndromes, and PGBH.
Differential diagnosis for hyperinsulinemic hypoglycemia after gastric bypass surgery
When differentiating endogenous from exogenous hypoglycemia, insulin and C-peptide levels are useful (Table 2). The pancreas produces proinsulin, which is broken down into insulin and C-peptide. Since exogenous insulin does not have a C-peptide component, people abusing insulin have elevated insulin levels with a low C-peptide level.11 Insulin secretagogues cause endogenous insulin secretion, resulting in elevated levels of both insulin and C-peptide. Thus, a screen for these medications is necessary to determine this as the cause.
Biochemical patterns and timing of hypoglycemia seen with endogenous and exogenous causes of hypoglycemia
Differentiating endogenous causes of hypoglycemia
Differentiating the endogenous causes (insulinoma, early or late dumping syndrome, and PGBH) can be challenging, as all 3 have similar biochemical profiles (Table 2).
Insulinoma is a tumor of pancreatic beta cells that produces excessive amounts of insulin. Unlike dumping syndrome, which only occurs postprandially, insulinoma primarily causes fasting hypoglycemia, although postprandial hypoglycemia can occur less commonly. Insulinoma after Roux-en-Y is rare. Only 7 cases have been reported.12
Dumping syndrome is classified as either early or late.
Early dumping syndrome usually occurs within 20 minutes of eating. The rapid transit of carbohydrates into the small intestine results in a fluid shift and a sympathetic response characterized by tachycardia, nausea, and diarrhea. Hypoglycemia is not present. Early dumping syndrome usually arises during the first few months after surgery.13
Late dumping syndrome usually occurs 1 to 4 hours after ingestion of a carbohydrate load, with symptoms of diaphoresis, dizziness, and fatigue caused by hypoglycemia from an excessive insulin release in response to the carbohydrates.13 It does not tend to cause neuroglycopenic symptoms.14 We define late dumping syndrome as postprandial hypoglycemic symptoms that occur after eating simple sugars and that resolve with dietary changes alone.
Differentiating late dumping syndrome from PGBH is difficult, as the line between the 2 processes is blurred.13
PGBH is postprandial hypoglycemia (although it can be fasting in severe cases), often with neuroglycopenic symptoms, that occurs despite adherence to an acceptable bariatric diet (outlined in Table 3). We categorize PGBH as mild, moderate, or severe. Mild PGBH resolves with dietary changes with or without an alpha-glucosidase inhibitor.
Dietary advice for patients after bariatric surgery
Moderate PGBH does not respond to an alpha-glucosidase inhibitor and dietary changes, and alternative or additional medication or medications are needed for resolution. Severe PGBH does not respond to dietary or medical interventions, and patients experience persistent episodes of neuroglycopenia.
THE EXACT MECHANISM IS UNCERTAIN
Patients with PGBH have a significant postprandial rise in glucose (often with levels > 200 mg/dL), leading to a robust insulin response and a subsequent drop in blood glucose.15
The exact mechanisms causing hypoglycemia are unknown, but excessive release of the incretin hormones glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP) are thought to contribute. GLP-1 is primarily secreted in the gut in response to nutrients, causing a glucose-dependent release of insulin and suppression of glucagon, as well as a delay in gastric emptying and motility. Salehi et al16 demonstrated excessive GLP-1 and insulin release after glucose administration in postbypass patients, with a more exaggerated response in those experiencing postprandial hypoglycemia.
Excessive incretin hormones may also contribute to pancreatic islet cell hyperplasia, leading to hyperinsulinism.17 Other proposed mechanisms of PGBH are the lack of a decrease in beta cell mass after gastric bypass, a postoperative increase in insulin sensitivity, a decrease in ghrelin (an insulin counterregulatory hormone), and an abnormal glucagon response.13,17
Pathologic changes vary widely
PGBH is a challenging diagnosis to make pathologically. On review of pancreatic tissue from 36 patients undergoing partial pancreatectomy for PGBH, the pancreatic islet cells of the PGBH group were larger and more irregular compared with controls.18,19 This histologic condition with islet-cell hypertrophy, hyperplasia, and other changes has been termed nesidioblastosis.11,14,10 However, the pancreatic tissue appears grossly normal. The histopathologic findings can vary greatly in individual cases and in one-third of cases the pancreatic changes can be minimal, so that “normal” and PGBH cells can be nearly impossible to distinguish from each other.11
As more gastric bypass procedures are performed, the incidence of PGBH will likely rise
DIAGNOSIS AND TREATMENT
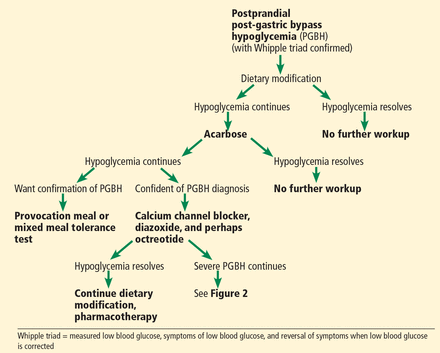
We recommend a stepwise approach to evaluating and treating PGBH (Figures 1 and 2).
Assessment and treatment of postprandial post-gastric bypass hypoglycemia (PGBH). See Figure 2 for assessment and treatment of fasting PGBH.
Assessment and treatment of fasting post-gastric bypass hypoglycemia (PGBH). See Figure 1 for assessment and treatment of postprandial pgbh.
Step 1: Evaluate blood glucose and Whipple triad
The first step is a thorough history, including food consumption and timing of hypoglycemic symptoms. Give the patient a glucometer to take home, with instructions to check blood glucose levels when hypoglycemic symptoms occur. The patient should keep a log documenting time tested, food consumed, symptoms, and blood glucose data.
Hypoglycemic symptoms are categorized as autonomic and neuroglycopenic. Autonomic symptoms include anxiety, palpitations, tremulousness, and diaphoresis. Neuroglycopenic symptoms include confusion, falls, seizures, and loss of consciousness.12
There are degrees of hypoglycemia and hypoglycemic symptoms. Clinical hypoglycemia—a blood glucose level low enough to cause signs or symptoms—can be confirmed by the Whipple triad:
Measured low blood glucose
Symptoms of low blood glucose
Relief of symptoms when low blood glucose is corrected.
Hypoglycemic symptoms can occur when the blood glucose level falls to less than 55 mg/dL in healthy people, but this cutoff can shift lower in someone who has recurrent hypoglycemia.
When the Whipple triad is documented, rule out nonhyperinsulinemic causes of hypoglycemia such as hypothyroidism, adrenal insufficiency, underlying organ dysfunction (ie, liver disease), and medications that cause hypoglycemia.
Step 2: Modify the diet
If postprandial hypoglycemia is occurring, the next step is dietary modification. Two studies showed that a low-carbohydrate diet prevented hypoglycemia; however, these diets contained nearly no carbohydrates (with meals consisting of eggs, sausage, cheese, and black coffee or tea).15,22
Instruct patients to never eat pure carbohydrates without fat or protein, as this can result in a more severe hypoglycemic response.22 In addition, foods with a high glycemic index (a measure of how a carbohydrate-containing food raises blood sugar) should be avoided, and a low glycemic index diet is recommended.23 High glycemic index foods include white bread, bagels, pretzels, and pineapple. Low glycemic index foods include 100% stone-ground whole wheat or pumpernickel bread, lima beans, butter beans, peas, legumes, lentils, and nonstarchy vegetables.
Our bariatric surgeons provide all postbariatric surgery patients with the dietary guidelines shown in Table 3.24 We also ask our patients with PGBH to limit carbohydrates to 15 to 30 g per meal and to limit added sugars to less than 4 g per meal, including regular and sugar alcohols (polyols). Snacks should contain only protein and fat. In severe cases, we further limit the diet to 15 g of carbohydrate per meal, with no added sugars.
The hypoglycemia occurring with PGBH is treated differently than the hypoglycemia that occurs in diabetic patients. Advise patients with PGBH to treat their hypoglycemic episodes with a simple sugar combined with a protein or fat (eg, a small handful of candy with a spoonful of peanut butter), as they will often have recurrent hypoglycemia if a simple sugar is used alone. If patients regain weight, ask them about frequent eating, which would be related to self-treatment of hypoglycemia.
Step 3: Start an alpha-glucosidase inhibitor
If postprandial hypoglycemia persists despite dietary modification, then start an alpha-glucosidase inhibitor such as acarbose. Acarbose inhibits carbohydrate absorption, resulting in a decreased insulin response; thus, it blunts the decline in postprandial blood glucose.
Unfortunately, gastrointestinal side effects such as flatulence, diarrhea, and abdominal pain occur in up to 20% of patients who take acarbose, often leading to its discontinuation.25 To minimize gastrointestinal side effects, we usually start with 25 mg of acarbose with 1 meal daily for 1 week, then increase the dosage weekly to 25 mg with the other 2 meals. If tolerated, acarbose can be increased to 50 to 100 mg with 3 meals daily.
Severe PGBH does not respond to any dietary or medical intervention
Step 4: Obtain a mixed meal tolerance test or a provocation meal test
If dietary changes and an alpha-glucosidase inhibitor do not prevent postprandial hypoglycemia from recurring, then confirmation of PGBH is needed, using a mixed meal tolerance test or a provocation meal test.
In a mixed meal tolerance test, the meal consists of 55% carbohydrate, 30% fat, and 15% protein. Patients with hyperinsulinemic hypoglycemia have a rapid rise in blood glucose (> 200 mg/dL) with a robust insulin response that is often followed by hypoglycemia after ingesting a meal containing carbohydrates in this test. Insulin levels that remain elevated after the plasma glucose level falls to less than 55 mg/dL indicate hyperinsulinism.11
Nevertheless, a mixed meal tolerance test will not always induce hypoglycemia. In a study of 51 patients with PGBH, all wore a continuous glucose monitor, were instructed to follow their normal diet for 5 days, and then underwent a mixed meal tolerance test on day 6. The glucose monitor revealed hypoglycemia in 75% of patients, while the mixed meal tolerance test was positive in only 29%.5
Moreover, to date, there is no standardized mixed meal.5,15 This might also explain the difference in prevalence of hypoglycemia detected by this test.
Based on these conflicting findings, we recommend a provocation meal test—ie, the patient is given foods that have induced hypoglycemia earlier.
Of note, the Endocrine Society guidelines on hypoglycemia state that an oral glucose tolerance test should never be used to document postprandial hypoglycemia.26 Lev-Ran and Anderson27 found that an oral glucose tolerance test could be positive in at least 10% of normal people.
Step 5: Consider other pharmacotherapy
For moderate to severe PGBH in which dietary modification and acarbose have failed, additional medical therapy is the next step. Medical therapies include calcium channel blockers, somatostatin analogues (eg, octreotide), and diazoxide.
Calcium channel blockers inhibit insulin release from beta cells28 but at the risk of hypotension. Mordes and Alonso29 treated 6 PGBH patients with nifedipine or verapamil with or without acarbose, and symptoms resolved in 5 of the 6 patients.
When we treat PGBH, we often add a calcium channel blocker as the next step in therapy if the patient has hypertension or if the blood pressure can tolerate this. If the patient’s blood pressure is low, then avoiding calcium channel blocker therapy may be necessary. The next step would be octreotide and then diazoxide.
Somatostatin analogues such as octreotide inhibit GLP-1 and insulin release.30 The most common side effects of octreotide are diarrhea and abdominal pain. Bile stone formation can also occur, but this is not common.
Diazoxide opens adenosine triphosphate-sensitive potassium channels and reduces the opening of calcium channels, inhibiting insulin release and raising blood glucose. In a study of 6 Japanese patients with inoperable insulinoma, diazoxide was used to treat hypoglycemia.31 Unfortunately, the doses required to control the low blood sugars also led to adverse reactions, most of which involved edema secondary to volume overload and other heart failure symptoms. Diazoxide also commonly causes hypotension and hirsutism.
Step 6: 72-hour fast
A 72-hour fast is recommended in severe cases of PGBH in patients for whom dietary modification and the additional pharmacotherapy outlined in step 5 have failed. A 72-hour fast is always indicated in evaluating confirmed fasting hypoglycemia. People with insulinoma usually have fasting hypoglycemia, while patients with dumping syndrome do not. Patients with PGBH usually do not have fasting hypoglycemia, but they can in severe cases.11
For safety, this test should be done in the hospital. Baseline plasma levels of insulin, C-peptide, proinsulin, beta-hydroxybutyrate, and glucose should be obtained. The patient then fasts, consuming only noncaloric and noncaffe inated beverages for 72 hours. During this time, capillary glucose checks are performed every 6 hours. If the capillary glucose level falls below 55 mg/dL,11,26 then the baseline tests are redrawn along with a sulfonylurea screen. To reduce costs and unnecessary testing, the tests are not sent for laboratory processing unless the plasma glucose is less than 55 mg/dL.
When the plasma glucose is less than 55 mg/dL, insulin production should cease. Elevated insulin levels and insulin byproducts raise concern for hyperinsulinism. These values confirm hyperinsulinemic hypoglycemia26:
Glucose < 55 mg/dL
Insulin ≥ 3 µU/mL
C-peptide ≥ 0.2 nmol/L
Proinsulin ≥ 5.0 pmol/L.
With recurrent hypoglycemia, the threshold for symptoms can shift lower
After hypoglycemia is confirmed, 1 mg of glucagon is given intravenously, and plasma glucose levels are obtained at 10, 20, and 30 minutes.11,26 A rise in plasma glucose of at least 25 mg/dL after intravenous glucagon injection indicates hypoglycemia due to hyperinsulinemia. Two-thirds of patients with insulinoma experience hypoglycemia within the first 24 hours, and nearly all experience hypoglycemia within 48 hours.26
Step 7: Obtain pancreatic imaging
If fasting hypoglycemia is present and hyperinsulinemic hypoglycemia is confirmed during a 72-hour fast, then pancreatic imaging should be obtained to evaluate for an insulinoma. We also recommend pancreatic imaging to rule out insulinoma when severe PGBH has not responded to dietary modification or pharmacotherapy.
Imaging is not recommended in PGBH that has been successfully treated with dietary modification with or without pharmacotherapy.
Endoscopic ultrasonography alone has 80% to 92% sensitivity for localizing a pancreatic mass as small as 5 mm. However, when coupled with computed tomography or magnetic resonance imaging, the sensitivity increases to nearly 100%.12
Step 8: Selective arterial calcium stimulation test
If a patient is found to have hyperinsulinemic hypoglycemia during a 72-hour fast but pancreatic imaging is negative, then selective arterial calcium stimulation testing (SACST) and hepatic vein sampling should be performed. Also, for severe PGBH, in which hypoglycemia has persisted despite dietary modification and pharmacotherapy, SACST can be performed to evaluate for possible localization of hyperinsulinism in patients considering surgery. For mild and moderate cases of PGBH, in which the hypoglycemia has been successfully treated with dietary changes with or without pharmacotherapy, SACST is not necessary.
This test can localize the area of excess insulin production in the pancreas in patients with an insulinoma. Patients with severe PGBH usually have diffuse hyperinsulinism without localization on SACST.32,33
When SACST is performed, a sampling catheter is placed in the femoral vein. Calcium gluconate is injected into the major arteries of the pancreas (superior mesenteric, gastroduodenal, and splenic arteries). Calcium stimulates release of insulin from an insulinoma or hyperplastic beta cells. Resultant insulin levels are measured in the hepatic vein. If there is a greater than twofold increase in insulin release from 2 segments, then the test is considered positive.
Thompson et al34 documented that insulin release from insulinoma is almost 4 times higher than in diffuse nesidioblastosis. SACST has a sensitivity of 96% for detecting insulinomas.35
Step 9: Other alternatives and surgery
In patients with severe PGBH for whom dietary modification and all pharmacotherapy have failed and who continue to have debilitating neuroglycopenia, there are options before proceeding with surgery, the last resort in this condition.
Continuous glucose monitoring is helpful in many patients with severe PGBH. Many of them have hypoglycemia unawareness, and the monitor alerts them when their blood sugar is low. In addition, the monitor indicates when the blood sugar is dropping, so that intervention can occur before hypoglycemia occurs.
Unfortunately, insurance coverage for continuous monitors in this patient population is limited. We argue that insurance should cover the cost for these severe cases.
Pasireotide, a somatostatin analogue that is longer-acting than octreotide, is approved for use in Cushing disease and acromegaly and actually causes hyperglycemia. In a case report of a 50-year-old woman, pasireotide resulted in less hypoglycemia and higher glucagon levels then octreotide.36 Pasireotide is available from Novartis for compassionate use in patients with severe PGBH.
Glucocorticoids are another off-label option. However, in excess, they can lead to iatrogenic Cushing syndrome, which has its own complications. Prednisone and diazoxide have been used together to help prevent hypoglycemia in a patients with inoperable insulinoma.31
Tube feeding. Some researchers have studied altering nutrition access through surgical means. McLaughlin et al37 discussed a case of gastric tube insertion into the remnant stomach of a patient with PGBH, with resolution of hypoglycemic symptoms and hypoglycemia; however, this does not always provide complete resolution of symptoms.37,38 If gastric bypass reversal is being considered, a trial of solely remnant stomach tube feeds (with no oral intake) should be pursued first. If this ameliorates the hypoglycemia, then gastric bypass reversal may be of benefit.
Surgery is the last resort if all of the above treatments have failed and severe debilitating neuroglycopenia persists. However, surgery poses risks, and the success rate in correcting hypoglycemia is not ideal. Surgical options include Roux-en-Y reversal, gastric pouch resection, and pancreatic resection.
In a review by Mala,2 75 patients with documented PGBH underwent surgical therapy. Hypoglycemic symptoms resolved in 34 of 51 pancreatic resections, 13 of 17 Roux-en-Y reversals, and 9 of 11 gastric pouch resections. However, the follow-up period was short.
As noted above, we recommend calcium stimulation testing only for severe cases of PGBH when surgery is being considered to evaluate for possible localization of hyperinsulinism for which partial pancreatectomy would be of benefit. Since there is no localization in many PGBH cases and the success rates are slightly higher in gastric bypass reversal, bypass reversal is usually preferred over partial or complete pancreatectomy.2,32,33
POTENTIAL FUTURE THERAPIES
Given the elevated GLP-1 levels and robust insulin response to glucose observed in PGBH, blocking GLP-1 may provide clinical benefit. Salehi et al16 found that a GLP-1 antagonist prevented surges in GLP-1 and reduced hypoglycemic episodes in patients with PGBH. Unfortunately, the medication they used was given as a continuous infusion and is not currently available.
Conversely, a GLP-1 agonist showed benefit in a series of 5 cases of PGBH.39 In addition, an insulin receptor antibody is undergoing phase 2 trials and has been shown to reverse insulin-induced hypoglycemia in rodents and humans; it may be a novel therapy in the future for hyperinsulinemic hypoglycemia.40
MORE STUDY NEEDED
As the prevalence of obesity continues to rise and more people opt for bariatric surgery for weight loss, we will likely continue to see an increase in PGBH, since the onset of PGBH can be delayed for many years after surgery.28
Unfortunately, the disease process involved in PGBH is not well understood. For example, we do not know why GLP-1 elevations or a robust insulin response causing hypoglycemia occurs in some but not all gastric bypass patients. Study is needed to elucidate the pathophysiology to further understand why most patients have no hypoglycemia after gastric bypass, some have mild to moderate PGBH, and a small percentage have severe PGBH with debilitating neuroglycopenia unresponsive to dietary changes and medications.
- Copyright © 2017 The Cleveland Clinic Foundation. All Rights Reserved.