ABSTRACT
Experts now recommend that all Helicobacter pylori infections be eradicated unless there are compelling reasons not to. As with other infectious diseases, effective therapy should be based on susceptibility.
We recommend clinicians have 2 first-line options to accommodate prior antibiotic use or drug allergy.
We recommend 4-drug combinations as first-line treatments, ie, either concomitant therapy or bismuth-containing quadruple therapy, to be taken for 14 days.
Concomitant therapy consists of the combination of amoxicillin, metronidazole, clarithromycin, and a proton pump inhibitor.
Bismuth quadruple therapy consists of the combination of bismuth, tetracycline, metronidazole, and a proton pump inhibitor.
After 2 treatments have failed, therapy with different regimens should be based on susceptibility testing.
Helicobacter pylori infection is an infectious disease and should be treated like one, with due consideration of antibiotic resistance and stewardship.1–4
This was the consensus of the 2015 Kyoto H pylori conference,2 and it signaled a fundamental shift in thinking. Up to now, H pylori treatment has not been based on infectious disease principles, leading to suboptimal results and antibiotic resistance. In addition, the conference recommended that H pylori infection be treated whenever it is found unless there are compelling reasons not to.
Here we review current and possible future regimens for eradicating H pylori that we hope will be more effective and will lead to less resistance than in the past.
H PYLORI AS AN INFECTIOUS DISEASE
Not until the late 1980s was H pylori recognized as the cause of peptic ulcer disease, which until then accounted for hundreds of thousands of hospitalizations and more than 100,000 surgical procedures each year.5 Now, peptic ulcer disease is routinely treated by eradicating H pylori. In addition, the World Health Organization has recommended considering H pylori eradication to reduce the risk of gastric cancer,6 which causes 738,000 deaths worldwide per year.7
The problems of how to diagnose and treat H pylori infection were taken on by gastroenterologists, and not by specialists in infectious disease.1 Even now, almost all the major reviews and consensus statements on H pylori come from gastroenterologists and are published in gastroenterology journals.2,8,9
But infectious diseases differ from most gastrointestinal diseases. In gastrointestinal problems such as constipation or inflammatory bowel disease,10 the causes are generally unknown, and there is a large placebo response to therapy. In contrast, in infectious diseases, the cause is generally known, there is no placebo response, and treatment success depends on susceptibility of the organism. Failure of proven regimens is generally due to resistant organisms, poor adherence, or, in the case of H pylori, poorly designed regimens in terms of doses, frequency of administration, or duration of therapy.
The differences extend to clinical trials of treatment.3 In other infectious diseases, treatment is based on susceptibility. The usual comparative approach in infectious diseases is a noninferiority trial in which the new treatment is compared with standard care, ie, a regimen that reliably achieves nearly 100% cure rates. Not so with H pylori. Most trials of H pylori therapy compared regimens in populations with high but unknown prevalences of resistance and therefore are of limited or no help to the clinician in choosing the best regimen for an individual patient.3
Many thousands of H pylori-infected patients participated in clinical trials in which the results would have been predictable if the researchers had assessed susceptibility before giving the drugs.11–13 Worse, many patients were also randomized to receive regimens that the investigators knew provided poor cure rates in the population being studied. This knowledge was generally not shared with the patients. This approach was used to demonstrate that a new regimen was superior to an old one, even though the new one was already known to be less affected by resistance to the key element in the comparator.
Clinicians generally do not test for susceptibility when treating H pylori, one reason being that such testing is often unavailable.3 However, almost every hospital, clinic, and major laboratory in the world provides susceptibility testing for other common local pathogens. H pylori is easy to grow, and laboratories could test for susceptibility if we asked them to.
Current H pylori recommendations may also contribute to the global increase in antimicrobal resistance.
As discussed below, all recent guidelines have recommended 4-drug non-bismuth-containing concomitant therapy as first-line therapy. An infectious disease colleague described it as a “hope therapy” because the prescriber hoped that the infection would be susceptible to either metronidazole or clarithromycin. All who receive this combination receive an antibiotic they do not need. This is an expedient rather than a medically rational choice resulting from failure to deal with H pylori as an infectious disease.
H PYLORI THERAPIES
Conceptually, treating infectious disease is straightforward: one should prescribe antimicrobial drugs to which the organism is susceptible3 (Table 1). However, clinical success lies in the details, which include the doses, frequency of doses, duration of therapy, timing of doses in relation to meals, and use of adjuvants such as antisecretory drugs, antacids, and probiotics. A number of regimens reliably yield high cure rates—95% or higher—if the organism is susceptible and the patients are adherent.
Recommended regimens for Helicobacter pylori
The effectiveness of any regimen may vary depending on the population it is used in, due to polymorphisms in drug-metabolizing enzymes such as CYP2C19.
Sequential therapy is obsolete
Sequential therapy for H pylori infection consisted of amoxicillin plus a proton pump inhibitor for 7 days, followed by clarithromycin, tinidazole, or metronidazole plus a proton pump inhibitor for a further 7 days. This regimen should not be used any more because concomitant therapy will always be superior (see below).
Need for 14 days of therapy
H pylori occupies a number of different niches in the body ranging from gastric mucus (which is technically outside the body) to inside gastric epithelial cells. As a general rule, 14-day therapy provides the best results, in part because the longer duration helps kill the organisms that persist in different niches.14,15
In addition, proton pump inhibitors, which are part of all the currently recommended regimens, require 3 or more days to reach their full antisecretory effectiveness, which further limits the effectiveness of short-duration therapies.
Shorter regimens should be used only if they are proved to be as good as 14-day regimens and if both achieve 95% or greater cure rates with susceptible infections.
How to choose a therapy
Since rational infectious-disease therapy is based on susceptibility, one should start by considering the susceptibility pattern in the local population and, therefore, the likely susceptibility in the patient in front of us.
Unfortunately, we do not yet have local or regional susceptibility data on H pylori for most locales. Until those data are available, we must use the indirect information that is available, such as the patient’s history of antibiotic use.
Triple therapy should not be used empirically
Triple therapy (Table 1) consists of the combination of:
Clarithromycin or metronidazole or a fluoroquinolone
Amoxicillin
A proton pump inhibitor.
However, prior use of a macrolide (eg, erythromycin, clarithromycin, or azithromycin), metronidazole, or a fluoroquinolone (eg, ciprofloxacin, levofloxacin) almost guarantees resistance to those drugs. In the United States, resistance to clarithromycin, metronidazole, levofloxacin, and related drugs is already wide- spread, and none should be used empirically in triple therapies. In contrast, amoxicillin, tetracycline, and furazolidone can often be used again, as resistance to them is rare even with prior use.
For example, 14 days of clarithromycin triple therapy (clarithromycin, amoxicillin, and a proton pump inhibitor) can be expected to cure more than 95% of patients who have susceptible infections and about 20% of those with resistant infections.16 This 20% is due to the proton pump inhibitor and amoxicillin, as the contribution to the cure rate from clarithromycin is close to zero.
If the prevalence of resistance to clarithromycin is 25%, the cure rate in the entire population will be a little more than 75%—97% in the 75% of the population with susceptible infections and 20% in patients who previously received clarithromycin (Figure 1).
Nomogram of expected rates of cure (vertical axis) with triple therapy (ie, either clarithromycin or metronidazole, plus amoxicillin, plus a proton pump inhibitor) for Helicobacter pylori infection if the prevalence of resistance to clarithromycin or metronidazole in the population (horizontal axis) is 20% (A), 40% (B), or 8% (C). Even if the prevalence of resistance to the clarithromycin or metronidazole component of the regimen is 100% (far right side of graph), the amoxicillin and proton pump inhibitor components of the regimen can be expected to cure approximately 20% of cases. A cure rate of at least 90% is desirable.
Based on Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: effect of resistance, duration, and CYP2C19 genotype. Helicobacter 2015; 21:85-90.
If we know that our patient has an infection that is susceptible to clarithromycin, metronidazole, or levofloxacin, good results could be achieved with triple therapy that includes a proton pump inhibitor, for 14 days. Fluoroquinolones have a number of black-box warnings from the US Food and Drug Administration (www.fda.gov/Drugs/DrugSafety/ucm500143.htm) and should always be a last choice. However, in the United States, lacking definite data about susceptibility to clarithromycin, metronidazole, and levofloxacin, we should assume resistance is present and use a 4-drug regimen (eg, concomitant therapy or bismuth quadruple therapy).
Concomitant therapy is preferred
Concomitant therapy is the combination of:
Amoxicillin
Metronidazole
Clarithromycin
A proton pump inhibitor.
Functionally, this is a combination of clarithromycin and metronidazole triple therapies, given simultaneously.17 The premise is that even though the prevalence of metronidazole resistance in the United States is high (20%-40%), and so is the prevalence of clarithromycin resistance (about 20%), the prevalence of resistance to both drugs at the same time is expected to be low (eg, 0.4 × 0.2 = 0.08, or 8%) unless the drugs had previously been used together, as in some older regimens that contained both. Thus, the metronidazole will kill the clarithromycin-resistant but metronidazole-susceptible strains, and the clarithromycin will kill the clarithromycin-susceptible, metronidazole-resistant strains. Only with dual resistant strains will this regimen fail (with a 20% cure rate due to the proton pump inhibitor and amoxicillin and a population cure rate of slightly more than 90%).
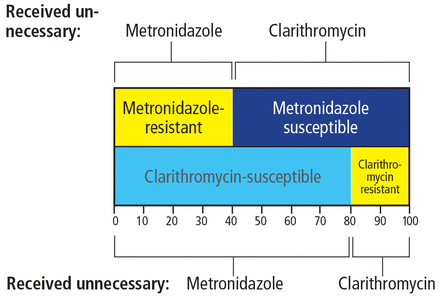
The downside of this highly recommended therapy is that all who receive it are getting an antibiotic that they don’t need, which is, in a global sense, inappropriate. In other words, all those who are cured by clarithromycin also receive metronidazole, which plays no role in treatment success, and those cured by metronidazole receive unneeded clarithromycin (Figure 2). Had susceptibility testing been available, those with susceptible strains would have received appropriate triple therapies, and those with dual resistance would not have received either antibiotic.
The “dirty little secret” of concomitant therapy (the combination of amoxicillin, metronidazole, clarithromycin, and a proton pump inhibitor) for Helicobacter pylori infection is a high rate of unnecessary antibiotic use. Shown are rates of unnecessary antibiotic use in a population with 20% clarithromycin resistance, 40% metronidazole resistance, and 8% dual resistance.
Thus, while we recommend concomitant therapy as an empiric regimen in populations that do not have high levels of resistance to metronidazole or clarithromycin (as those would also have a high prevalence of dual resistance), one must be aware of the “dirty little secret” of inappropriate antibiotic use that accompanies it and some other H pylori therapies (eg, vonoprazan triple therapy in Japan).18–20
Bismuth quadruple therapy is an alternative
Bismuth quadruple therapy (Table 1) consists of:
Bismuth
Tetracycline
Metronidazole
A proton pump inhibitor.
This was the first truly effective regimen for H pylori. Its advantage is that it can partially or completely overcome metronidazole resistance.21,22 As such, it is potentially ideal, as it should be effective despite resistance to clarithromycin, metronidazole, or levofloxacin, and it can be used in patients allergic to penicillin.
The major downside is a high frequency of side effects, particularly abdominal pain, nausea, and vomiting, often resulting in poor adherence. Most regimens that contain antibiotics have side effects, but adherence seems to be more of a problem with bismuth quadruple therapy, probably because of the combination of the high doses of metronidazole and tetra- cycline.22 In our experience, this regimen can be effective if the physician takes the time to explain to the patient that side effects are common but treatment success depends on completing the full course of 14 days.
Another problem is that tetracycline has become difficult to obtain in many areas, and doxycycline cannot be substituted in those with metronidazole resistance. To date, it has been difficult or impossible to obtain the same excellent results with doxycycline as can be obtained with tetracycline. It is not clear why.21
To use bismuth quadruple therapy one must often use a name-brand product, Pylera. Pylera is packaged as a 10-day course, which is effective against metronidazole-susceptible infections. However, 14 days are generally required to achieve a high cure rate with metronidazole-resistant infections, which are the main indication for use of this product. Moreover, Pylera does not include a proton pump inhibitor, which must be prescribed separately.
In the United States, Pylera is expensive, costing $740 to $790 with a coupon for a 10- day supply and proportionally more for the required 14-day supply (www.goodrx.com/pylera?drug-name=pylera), whereas in Europe it costs less than 70 Euros ($73).21 If generic tetracycline is available, the US cost for 14 days of generic bismuth quadruple therapy is less than $50.
An alternate and simpler approach is to substitute amoxicillin for tetracycline.23 This regimen has been used successfully in China and was shown to be noninferior to the tetracycline-containing regimen in a head-to-head comparison.24
Recent studies have confirmed earlier Italian studies suggesting that twice-a-day bismuth and tetracycline is effective, which would further simplify therapy and possibly reduce side effects.21,23,24 These variations on bismuth quadruple therapy have not yet been optimized to where one can reliably achieve 95% or greater cure rates, and further studies are needed.
Why include more than 1 antibiotic?
The H pylori load in the stomach is typically large, which increases the odds that a sub-population of resistant organisms is present. Resistance may be due to a relatively high rate of mutation in certain bacterial genes.25 This is particularly a problem with clarithromycin, metronidazole, and fluoroquinolones and is reflected in a high rate of resistance among patients for whom single-drug regimens have failed. These drugs are always given with a second antimicrobial to which H pylori rarely becomes resistant, such as amoxicillin or tetracycline.
Why include a proton pump inhibitor?
An antisecretory drug is needed to increase the gastric pH, which makes antimicrobial therapy more effective. It also decreases antibiotic washout from the stomach and likely protects and increases the gastric concentration of some antibiotics.
The activities of amoxicillin, fluoroquinolones, and to a lesser degree clarithromycin are pH-dependent. For example, keeping the gastric pH above 6.0 promotes H pylori replication,26,27 making it is more susceptible to amoxicillin (reviewed in detail by Dore et al21). A gastric pH of 6.0 or more is very difficult to achieve with proton pump inhibitors, and has been accomplished regularly only in people who metabolize these drugs slowly (“slow metabolizers”) who received both the proton pump inhibitor and amoxicillin every 6 hours for 14 days.21
With standard clarithromycin, metronidazole, or fluoroquinolone triple therapy, proton pump inhibitors appear to provide satisfactory cure rates when given for 14 days in standard doses. However, double doses (eg, 40 mg of omeprazole or an equivalent) may be slightly better, especially in the presence of resistance.
The cure rate reflects the sum of the 2 populations of organisms: the susceptible and the resistant. In triple therapy, increasing the gastric pH with a proton pump inhibitor makes the amoxicillin component of the regimen more effective against resistant organisms and thus increases the cure rate. For example, in Western countries, esomeprazole 40 mg (approximately equivalent to rabeprazole 40 mg, omeprazole or lansoprazole 60 mg, or pantoprazole 240 mg)28 given twice a day in a 14-day triple therapy regimen cures about 40% to 50% of resistant infections. This benefit will be evident in an improvement in cure rates in populations in which resistance has reduced the average cure rate. This is also why metaanalyses have shown better results with second-generation proton pump inhibitors and with longer duration of therapy.29,30
Generally, we recommend omeprazole 40 mg twice a day or an equivalent (Tables 1–3).
Recommended salvage regimens for Helicobacter pylori
Possible future regimens for Helicobacter pylori
Would a potassium-competitive acid blocker be better than a proton pump inhibitor?
Vonoprazan is a potassium-competitive acid blocker. It does not require intermediate complex formation and is stable at low pH. It has a longer half-life than proton pump inhibitors, and its bioavailability is unaffected by food.31 It was recently approved in Japan for H pylori eradication in combination with clarithromycin or metronidazole plus amoxicillin.18
Vonoprazan is more effective than current proton pump inhibitors for keeping the gastric pH high. There are no published studies of vonoprazan dual therapy in Western countries, but given twice a day for 7 days along with twice-daily amoxicillin it cured only approximately 80% of clarithromycin-resistant strains. Further studies are needed to identify the optimum proton pump inhibitor or potassium-competitive acid blocker, dose, and duration.
Misuse of antibiotics
In triple therapy, the second antimicrobial drug (eg, amoxicillin) is given in part to prevent resistance from developing. It is not clear whether the combination is additive or synergistic, but until we can reliably maintain the intragastric pH above 6.0, which would increase the effectiveness of the amoxicillin component of the regimen, this practice cannot be considered as misuse of antibiotics.
In contrast, in the 4-drug nonbismuth combinations (concomitant, sequential, and hybrid therapies) and the new vonoprazan, clarithromycin, and metronidazole triple therapies, 1 of the antibiotics provides no benefit to some, often most, of the patients.18–20,32 This practice should end when susceptibility data become more widely available and when vonoprazan becomes available, so that we can deliver effective vonoprazan-amoxicillin dual therapy.
First-, second-, and third-line therapies
Many recommendations give advice in terms of first-, second-, and third-line therapies. In practice, a physician should have at least 2 first-line regimens (a first and a second choice). Both should be proven highly successful as empiric therapies in one’s patient population but differ in terms of primary antibiotics. This approach allows the clinician to tailor therapy depending on whether he or she suspects antibiotic resistance (eg, if the patient has taken clarithromycin before) or the patient is allergic or cannot take 1 or more drugs.
Two treatment failures with 2 different regimens known to be effective suggest poor compliance (a difficult patient) or a multiple- drug-resistant infection (a difficult infection). That patient would require salvage therapy (Table 2), which logically should be based on antimicrobial testing or, at a minimum, consultation with someone who frequently deals with this problem.
Test of cure
Monitoring the outcome of therapy (testing for cure) is essential, as it provides a reliable measure of the local effectiveness of particular therapies and also serves as an early warning of development of resistance in one’s patient population.14
Unless there are compelling reasons, testing for cure should use noninvasive testing with the urea breath test or stool antigen test. It is recommended that this be delayed at least 4 weeks to allow the organisms if still present to repopulate the stomach sufficiently for the tests to become positive. Because antibiotics, bismuth, and proton pump inhibitors reduce the bacterial load, they should be withheld at least 2 weeks before testing. Histamine-2 receptor antagonists can be substituted for proton pump inhibitors if antisecretory therapy is needed for symptoms, and continued up to the day before testing. The urea breath test should contain citric acid to overcome any residual pH effects. Physician groups should share their experience so as to alert the community about which therapies should likely be avoided.33
Salvage therapy
Salvage therapy is given after 2 or more treatment failures with different antibiotics. Ideally, the regimen should be based on the results of antimicrobial testing. Current regimens include rifabutin triple therapy, dual therapy (a protein pump inhibitor or vonoprazan and amoxicillin), or furazolidone quadruple therapy (Table 2).
Furazolidone is a synthetic nitrofuran derivative that is effective against many enteric organisms, including gram-negative bacteria and protozoa. It is not available in most Western countries but is available in many other parts of the world.34,35 It is also a monoamine oxidase inhibitor and thus interacts with many drugs and foods (eg, soy sauce, aged cheeses), leading to a relatively high rate of side effects such as fever, palpitations, and skin rash.
Rifabutin-containing regimens, generally, a proton pump inhibitor, amoxicillin 1 g, and rifabutin 150 mg, all twice a day (Table 3) provide average cure rates of less than 80% (typically in the mid-70% range).36 Borody et al37 reported greater than 95% success with a 12-day regimen consisting of rifabutin 150 mg once daily (half-dose), amoxicillin 1.5 g 3 times a day, and pantoprazole 80 mg (approximately equivalent to omeprazole 20 mg) 3 times a day. Ciccaglione et al,38 in a small study, used a 10-day quadruple regimen containing a proton pump inhibitor, amoxicillin, rifabutin, and bismuth (all twice a day), with high cure rates. The results of these studies are yet to be confirmed, and the optimal rifabutin- containing regimen remains to be determined.
PROBIOTICS
There is considerable interest in using probiotics to enhance the effectiveness of antimicrobial therapy for H pylori by increasing tolerability, reducing side effects, and therefore improving compliance.39,40
In a meta-analysis of 14 randomized trials (N = 1,671), when probiotics were added, pooled H pylori eradication rates were only slightly improved: 83.6% (95% CI 80.5%–86.7%) with probiotics and 74.8% (95% CI 71.1%–78.5%) without probiotics by intent-to-treat analysis.41
Another meta-analysis of probiotics suggested that those containing Saccharomyces boulardii, Lactobacillus, and Bifidobacterium significantly increased the eradication rate of triple therapy in populations with high rates of antimicrobial resistance and reduced the risk of overall H pylori therapy-related adverse effects, especially diarrhea.42,43
At present, we recommend that probiotics be considered only for patients who are likely not to comply with treatment (eg, those with irritable bowel syndrome or difficulty taking antibiotics), to try to take advantage of their ability to improve antibiotic tolerability.
Footnotes
Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grants R01 DK062813 and DK56338 which fund the Texas Medical Center Digestive Diseases Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the Veterans Administration or National Institutes of Health.
Dr. Graham is a consultant for BioGaia, RedHill Biopharma, and Takeda Pharmaceutical Ltd.
- Copyright © 2017 The Cleveland Clinic Foundation. All Rights Reserved.