A 65-year-old man presented to the emergency department after having 3 black, tarry bowel movements in the last 24 hours. He had had another episode of melena 4 months earlier, in which upper endoscopy had revealed esophagitis.
For the past 3 years he had chronic anemia related to primary myelofibrosis. The diagnosis had been confirmed by bone marrow biopsy that showed increased cellularity (70%–80%), 1.4% blasts, myelofibrosis (graded MF-3 on a scale of MF-0 to MF-3), normal karyotype, BCR/ABL-negative, and JAK2-positive. He was being treated with epoetin alfa and ruxolitinib for this condition and needed blood transfusions every other week. His last transfusion had been earlier in the day of the bleeding, and his hemoglobin level at that time was 7.6 g/dL (reference range 13.2–16.6).
The patient also had hypertension, stage 3 chronic kidney disease, vertigo, and gout. Asked whether he was taking any drugs that could predispose to bleeding such as anticoagulants, antiplatelet agents, nonsteroidal anti-inflammatory drugs, or selective serotonin reuptake inhibitors, he said he was not.
PRIMARY MYELOFIBROSIS: A NEOPLASM OF BONE MARROW
1. Which of the following is the most common cause of death in patients with primary myelofibrosis?
Bleeding
Cardiovascular complications
Leukemic transformation
Infection
Primary myelofibrosis is a chronic myeloproliferative neoplasm.1 Its clinical manifestations are nonspecific and include fever, fatigue, weight loss, and night sweats. In the absence of symptoms, it is usually discovered in a workup for anemia, splenomegaly, or hepatomegaly.2 Primary myelofibrosis is classified as either overtly fibrotic or prefibrotic.
The World Health Organization lists 3 major and 5 minor criteria for diagnosis.2,3
Major criteria:
Megakaryocyte changes
JAK2, CALR, or MPL mutations or clonal markers on bone marrow analysis
Exclusion of other myeloid neoplasm diagnoses.
Minor criteria:
Anemia not otherwise explained
Leukocytosis
Palpable splenomegaly
Increased serum lactate dehydrogenase
A leukoerythroblastic blood smear, confirmed in 2 consecutive determinations.3
The diagnosis of overt primary myelofibrosis is established if all 3 major criteria and 1 minor criterion are met.2
Causes of death
The most common causes of death in patients with primary myelofibrosis are leukemic progression, responsible for up to 20% of deaths,2 followed by cardiovascular complications, infection, and bleeding.4
There are validated prognostic scoring tools for primary myelofibrosis. Age older than 65, severity of anemia, degree of leukocytosis, number of circulating blasts, and presence of constitutional symptoms are associated with worse survival rates.2
CASE CONTINUED: INITIAL EVALUATION
The patient was awake, oriented, and able to provide a coherent medical history. He appeared mildly malnourished but with a good general appearance. His pulse was 104 beats per minute and his blood pressure was 136/57 mm Hg. His spleen was palpable, and the rims of his conjunctiva were mildly pale. On rectal examination, his stool was black and tarry. No spider angiomas or jaundice was noted on physical examination, and there were no signs of ascites on abdominal examination.
Results of a complete blood cell count and other initial laboratory tests were as follows:
Hemoglobin 6.6 g/dL (reference range 13.2–16.6)
Hematocrit 20.8% (38.3%–48.6%)
Mean corpuscular hemoglobin 28.4 pg (25.4–32.7)
Red blood cell count 2.32 × 1012/L (4.35–5.65)
Red cell distribution width 26.9% (11.8%–14.5%)
White blood cell count 12.9 × 109/L (3.4–9.6)
Platelet count 210 × 109/L (135–317).
Glomerular filtration rate 55 mL/min/1.73 m2 body surface area (> 60)
Serum creatinine 1.39 mg/dL (0.74–1.35)
Total bilirubin 1.3 mg/dL (≤ 1.2)
Sodium 140 mmol/L (135–145)
Potassium 4.7 mmol/L (3.6–5.2)
Chloride 105 mmol/L (98–107)
Blood urea nitrogen 29 mg/dL (8–24)
Prothrombin time 17.5 seconds (11.6–14.7)
International normalized ratio 1.4 (0.8–1.1).
The patient received 2 units of packed red blood cells and an intravenous proton-pump inhibitor.
DOES THE PATIENT NEED TO BE ADMITTED?
2. Which of the following would not influence the decision whether to admit the patient to the hospital or perform an outpatient workup?
Melena
Low hemoglobin level
Tachycardia
Splenomegaly
Scoring systems are available to help determine the need for hospitalization in patients with upper gastrointestinal bleeding.
The Glasgow-Blatchford score is recommended by the International Consensus Group guideline.5 This 23-point score is based on sex, pulse, systolic blood pressure, blood urea nitrogen, hemoglobin, chronic liver disease, chronic heart disease, melena, and syncope.6 A score of 0 or 1 is associated with a very low risk of rebleeding, death, or need for urgent endoscopic intervention.7
The Rockall score, also commonly used,8 is based on clinical components (age, blood pressure, heart rate, and comorbidities) and endoscopic findings (diagnosis and bleeding stigmata). However, it is less accurate than the Glasgow-Blatchford score.9,10
The Glasgow-Blatchford score has been proven for assessing the need for intervention for both variceal11 and nonvariceal bleeding.12 Different studies confirmed its accuracy in predicting outcomes.7,10,13 A modified Glasgow-Blatchford score, which omits the subjective components (comorbidities, melena, and syncope), has also been shown to perform well.14 These scores do not consider splenomegaly.
The patient had no history of liver disease, heart failure, or recent syncope or presyncopal symptoms. His calculated Glasgow-Blatchford score was 12. He was admitted to the hospital for further evaluation and upper endoscopy.
CASE CONTINUED: UPPER ENDOSCOPY PERFORMED
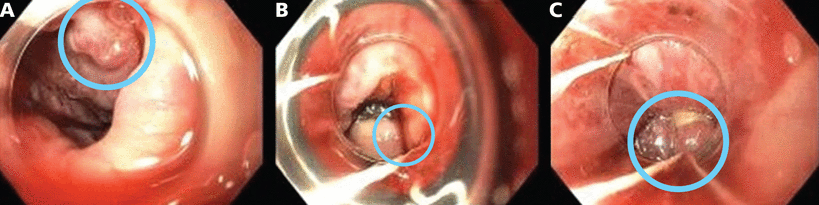
In view of the patient’s worsening anemia, upper endoscopy was performed. Findings included 5 columns of spurting, large varices (> 5 mm, grade 3 on a scale of 3) in the lower third of the esophagus (Figure 1A), and red, dilated venules (ie, the “red wale” sign). No varices were seen in the stomach, although there was a substantial amount of clotted blood in the gastric fundus and body, which could have hidden any varices there. Six bands were placed (Figures 1B, 1C), which partially eradicated the esophageal varices, and the patient was started on octreotide by continuous infusion for 72 hours. Intravenous ceftriaxone 1 g was administered approximately 4 hours after the patient presented to the emergency room, and he received 1 g daily on subsequent days. He had no further episodes of melena during the hospitalization, and he was discharged after 4 days.
Views from the patient’s initial upper endoscopy. (A) Large (grade 3) varices (circle) in the lower third of the esophagus. (B) Band placement in varices at the gastric cardia and gastroesophageal junction (circle). (C) Band placement in varices in the lower third of the esophagus (circle).
MANAGEMENT OF ESOPHAGEAL VARICES
3. Which option is the best next step in managing esophageal varices after hospitalization?
A beta-blocker and follow-up endoscopy in 1 to 4 weeks
Abdominal ultrasonography and repeat endoscopy in 1 week
A transjugular intrahepatic portosystemic shunt
Abdominal ultrasonography and upper endoscopy in 4 to 8 weeks
Hemostasis in esophageal variceal bleeding should preferably be achieved with variceal band ligation.10 Sclerotherapy should usually be avoided but may be necessary if visualization is difficult or if there is extensive scarring from previous banding.15
The management goals for a patient with acute variceal hemorrhage are to avoid complications and reduce the risk of rebleeding. Red, dilated venules (ie, the red wale sign) and are considered the endoscopic sign with the highest risk of rebleeding.16
Secondary prophylaxis after an episode of variceal bleeding can lessen the risk of death. Initial management includes noncardioselective beta-blockers such as propranolol, nadolol, and carvedilol. These medications reduce the risk of esophageal hemorrhage17 and slow the rate of growth of varices.18 The dosage should be titrated to the highest dose tolerated or to a reduction in the resting heart rate by 25% or as low as 55 beats per minute. Our patient was started on carvedilol 6.25 mg daily.
The American Association for the Study of Liver Diseases recommends upper endoscopy every 2 to 8 weeks until the varices are eradicated, again 3 to 6 months after eradication, and then every 12 months.19,20 Ultrasonography is recommended in the acute setting to investigate new or worsening ascites in patients with bleeding esophageal varices, if there is suspicion of portal vein thrombosis, or to investigate portal hypertension if there is no known history of liver disease.21
In patients with advanced cirrhosis (Child-Pugh class B or C), placement of a transjugular intrahepatic portosystemic shunt within the first 3 days after presentation was shown to reduce the risks of further bleeding and death at 1 year.18,22–25 In general, the major drawback of this treatment is overt hepatic encephalopathy, which occurs in about one-third of patients. It should be therefore reserved for select cases.26 In the setting of bleeding esophageal varices, the ideal candidate for shunt placement within the first 3 days of presentation would have no history of hepatic encephalopathy, no history of heart failure or tricuspid valve regurgitation, no signs of sepsis, no pulmonary hypertension, and no significant coagulopathy.
CASE RESUMED: FURTHER INVESTIGATION
Abdominal ultrasonography was done to evaluate for hepatic or portal vein thrombosis. The liver was normal in appearance, and blood flow was normal on Doppler studies. The spleen was markedly enlarged (measuring 24.5 cm), and there was mild ascites.
Four weeks after discharge, upper endoscopy was performed again and revealed several varices in the esophagus. The varices were medium-sized (grade 2 on a scale of 3) and not bleeding, and there were no stigmata of recent bleeding and no red wale signs. Three bands were placed in the distal 5 cm of the esophagus. The mucosa of the stomach was diffusely mildly congested, with apparent extrinsic compression along the greater curvature of the gastric body. In the second portion of the duodenum, the mucosa appeared swollen with the suggestion of “ropey” protuberances.
Since Doppler ultrasonography did not show any obvious portal vein thrombosis, magnetic resonance imaging of the abdomen was performed to evaluate the etiology of the esophageal varices. This showed cirrhotic liver morphology and hepatic iron deposition (likely related to transfusions or iron therapy), without suspicious liver lesions. The hepatic vasculature was patent, with conventional arterial anatomy and large main portal and splenic veins. Portal hypertension with moderate ascites and venous collaterals including gastroesophageal varices were present. The spleen was markedly enlarged, measuring 22.5 cm.
ETIOLOGY OF PORTAL HYPERTENSION
4. What is the most common cause of portal hypertension in the United States?
Idiopathic
Schistosomiasis
Extrahepatic portal venous obstruction
Neoplastic occlusion of the intrahepatic portal vein
Budd-Chiari syndrome
Cirrhosis
Cirrhosis accounts for most cases of portal hypertension in Western countries.27 Noncirrhotic causes account for less than 10% of cases, and they present with normal synthetic liver function and normal to mildly elevated hepatic venous pressure gradient.27,28 Common causes of noncirrhotic portal hypertension include Budd-Chiari syndrome, portal vein thrombosis, and idiopathic portal hypertension.29 Schistosomiasis, a disease caused by parasitic worms lodging in the liver, is common in Africa and other tropical areas but not in the United States.
Causes of portal hypertension are divided according to their anatomic location. Prehepatic and posthepatic causes differ from intrahepatic disorders in that they do not directly involve the liver. These disorders cause a disruption in the hepatic vascular system that leads to increased pressure in the portal venous system.
Intrahepatic causes are categorized according to where the obstruction is in relation to the sinusoids, ie, presinusoidal, sinusoidal, or postsinusoidal (Table 1), although many of them can affect more than one vascular compartment.30
Etiology of portal hypertension
Idiopathic portal hypertension has been reported worldwide, but mainly in developing countries, the Indian subcontinent, and Japan.31–33 By definition, the cause is unknown, but studies have found associations with other conditions including immunologic disorders, genetic diseases, human immunodeficiency virus infection, and exposure to drugs and toxins.29
Noninvasive diagnostic approaches such as liver imaging modalities are useful, but the differential diagnosis to determine the cause of the portal hypertension relies on thorough interpretation of the liver biopsy.34,35
CASE RESUMED: BIOPSY
The patient’s cirrhosis was presumed to be secondary to iron deposition. Results of iron studies were as follows:
Ferritin level 1,227 µg/L (24–336)
Iron 156 µg/dL (50–150)
Total iron binding capacity 165 µg/dL (250–400)
Percent saturation > 90% (14%–50%).
However, the patient’s levels of liver enzymes and albumin were within normal limits. We decided to confirm the diagnosis of cirrhosis and further investigate its etiology with pathologic examination.
Transjugular liver biopsy was performed, and during the procedure the corrected sinusoidal pressure gradient was 10 mm Hg, indicating sinusoidal portal hypertension. Pathologic study of the sampled tissue revealed extramedullary hematopoiesis, ie, production of blood cells outside of the bone marrow, and no significant fibrosis on trichrome staining. The combination of portal hypertension with patent portal and hepatic veins and no cirrhosis on liver biopsy led to the diagnosis of noncirrhotic intrahepatic portal hypertension.
EXTRAMEDULLARY HEMATOPOIESIS
5. What are the organs most often affected by extramedullary hematopoiesis?
Liver and spleen
Kidney and adrenal glands
Lungs and pleura
Breasts
Patients with primary myelofibrosis can develop anemia and hepatosplenomegaly due to ineffective erythropoiesis and extramedullary hematopoiesis.36
In extramedullary hematopoiesis, hematopoietic stem cells escape from the bone marrow, travel through the bloodstream, and infiltrate other organs.37 In most reported cases, the organs affected were the liver and spleen,38,39 but extramedullary hematopoiesis can also affect the kidneys, adrenal glands, lymph nodes, lungs, pleura, skin, breasts, dura mater, ovaries, thymus, gastrointestinal tract, and central nervous system.37
In the liver, hematopoietic cells obstruct the sinusoids, resulting in portal hypertension.37,40 This can be managed by placing a transjugular portosystemic shunt to alleviate the symptoms,36 especially in patients presenting with recurrent variceal bleeding and refractory ascites.2
FURTHER MANAGEMENT AND OUTCOME
On portal pressure measurement, the corrected sinusoidal gradient was a mean of 10 mm Hg, indicating sinusoidal portal hypertension. Portal hypertension has been described in patients with myelofibrosis. The cause is not clear, but it has been postulated that this is due to liver infiltration from extramedullary hematopoiesis and fibrosis.
Therefore, the hematology care team decided to perform allogeneic stem cell transplant. On post-transplant day 12, the patient’s course was complicated by cytokine release syndrome, frequent diarrhea, hyperbilirubinemia with interval development of ascites, and stress cardiomyopathy. He was admitted to the intensive care unit for management of septic shock secondary to candidemia and Candida krusei peritonitis. Sepsis was further complicated by acute hypoxic respiratory failure, acute kidney injury, severe pulmonary edema, and episodes of pulseless electrical activity. After a conversation with the family, the patient was taken off life-support and pronounced dead.
TAKE-HOME POINTS
All patients with gastrointestinal bleeding should be thoroughly evaluated.
The Glasgow-Blatchford score is a useful tool to assess the need for hospitalization in patients with gastrointestinal bleeding.
In a patient with esophageal varices and no history of liver disease, noncirrhotic causes of portal hypertension should be considered.
Secondary prophylaxis after esophageal variceal bleeding is important to improve outcomes and reduce mortality.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.