A 59-year-old woman was hospitalized after 3 days of neck pain, painful swallowing, headache, and fever. Three years earlier she had received a liver transplant because of chronic liver disease due to hepatitis C, and afterward had contracted posttransplant lymphoproliferative disease. Because she needed frequent blood tests, a subclavian port had been placed 7 months before the current presentation and last accessed 6 weeks ago. She also had stage 3 chronic kidney disease. She had no history of thyroid disease or alcohol or tobacco use.
Her medications at home included the following:
Tacrolimus 1 mg by mouth twice a day
Oxycodone 30 mg by mouth every 6 hours as needed
Oxymorphone 40 mg by mouth every 12 hours as needed
Modafinil 200 mg by mouth as needed
Promethazine 12.5 mg by mouth as needed
A multivitamin, fish oil, vitamin D, and calcium supplements daily.
On examination, her temperature was 38.7°C (101.7°F), heart rate 103 beats per minute, blood pressure 129/64 mm Hg, and respiratory rate 22 breaths per minute. She was alert and oriented and answered questions appropriately.
Her neck was tender to palpation all over but particularly in the left anterior area. There were no palpable masses or swollen glands or lymph nodes. The area around the subclavian port was red and tender. Cardiovascular and pulmonary examinations were normal. She had a surgical scar on the abdominal wall. The rest of the abdominal examination was normal.
Initial laboratory results are listed in Table 1.
The patient’s initial laboratory results
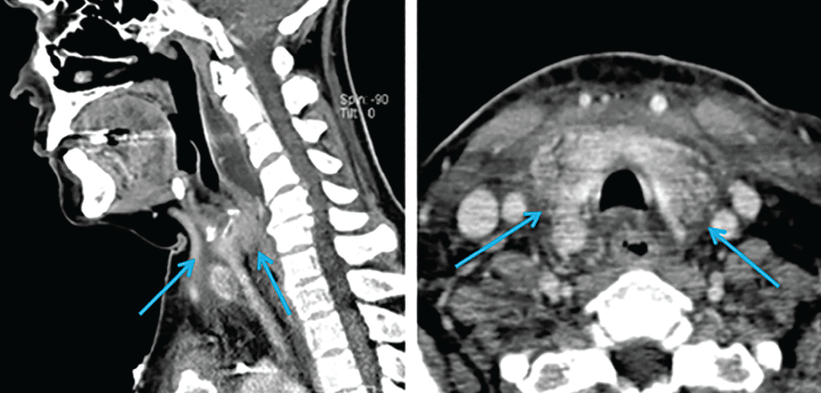
Contrast-enhanced computed tomography (CT) of the head and neck revealed soft-tissue inflammation tracking from the left anterior chest wall, encompassing the thyroid, and reaching into the retropharyngeal space (Figure 1).
Sagittal (left) and horizontal (right) views on computed tomography demonstrate inflammatory changes arising from the chest and tracking superiorly along the neck (arrows).
Blood cultures from the subclavian port grew methicillin-resistant Staphylococcus aureus after 15 hours, as did cultures from the peripheral blood after 30 hours.
Improvement, then a turn for the worse
The team removed her subclavian port, started intravenous vancomycin, and admitted her to the hospital. Three days later, contrast-enhanced CT showed marked improvement: the thyroid gland was smaller, and the inflammatory fat-stranding previously seen surrounding the gland had resolved.
However, on the patient’s fourth day in the hospital, she became increasingly short of breath, confused, agitated, and anxious. She had no focal neurologic deficits, but her mental status waxed and waned, with intermittent delirium and loss of orientation to time.
Her temperature was still 38.4°C (101.1°F), but her heart rate had risen to 153 beats per minute, blood pressure 153/107 mm Hg, and respiratory rate 33 breaths per minute. Other new findings on physical examination were the following:
Fine inspiratory crackles at the bases of both lungs
Eyelid lag (the top eyelids remaining high when the patient looks down)
Pitting edema in both ankles, rated 1+ (mild) on a scale of 4
Generalized hyperreflexia.
Electrocardiography revealed sinus tachycardia without ST-T-wave changes. Her white blood cell count was 12.8 × 109/L (reference range 3.4–9.6) with 78% neutrophils (reference range 40%–60%). Trans-thoracic echocardiography revealed an ejection fraction of 45% but no wall-motion abnormalities. CT angiography of the chest was negative for pulmonary embolism.
DIFFERENTIAL DIAGNOSIS
1. Which one of the following conditions is the most likely diagnosis?
Thyrotoxicosis, thyroid storm
Pheochromocytoma
Adrenal crisis
Delirium tremens (withdrawal from heavy alcohol use)
Pheochromocytoma can cause many symptoms similar to those of thyroid storm, but they are often paroxysmal and brief. A systematic review by Soltani et al1 found that headache, which our patient did not have, was the second most common symptom of pheochromocytoma (after hypertension), with a pooled sensitivity of 60.4% among 25 studies. Absence of the classic triad of headache, tachycardia, and diaphoresis had a negative likelihood ratio of 0.139 (95% confidence interval 0.059–0.331) for the diagnosis of pheochromocytoma.
Further, the patient had symptoms that are not common in pheochromocytoma such as eyelid lag, hyperreflexia, altered mental status, and lower extremity edema, overall making this diagnosis less likely.
Adrenal crisis can also present with nonspecific signs such as fever, confusion, and tachycardia. However, patients with adrenal crisis commonly have low blood pressure (not high, as in this patient) worsened by dehydration due to vomiting and diarrhea. Other features of adrenal crisis not seen in this patient are lethargy (not agitation) and a constellation of laboratory abnormalities (eg, hyperkalemia, hypercalcemia, hypoglycemia, hyponatremia). Also, this patient had not received glucocorticoids in the near past, which would have suggested adrenal insufficiency from steroid withdrawal.
Delirium tremens is not likely without other features of alcohol use disorder or withdrawal: nausea, vomiting, diaphoresis, tremors, fatigue, pallor, and mydriasis, and laboratory findings such as elevated aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase, carbohydrate-deficient transferrin, and mean corpuscular volume, a mildly low platelet count, and hypomagnesemia.
Thyroid storm should be suspected in this patient in view of her rapid deterioration and findings that suggest thyrotoxicosis such as altered mental status, confusion, anxiety, sinus tachycardia, hyperthermia, hypertension, tachypnea, pulmonary crackles (likely due to pulmonary edema from acute heart failure), lower extremity pitting edema, eyelid lag, and hyperreflexia.
HYPERTHYROIDISM AND THYROID STORM
Hyperthyroidism is a general term that means the thyroid gland is producing thyroid hormone above normal levels.
Thyrotoxicosis is the manifestation of excessive concentrations of circulating thyroid hormone in the body due to any cause.
Thyroid storm is extreme thyrotoxicosis with physiologic decompensation resulting in severe multisystem dysfunction, often to the point of failure. Because mortality rates range from 20% to 30%,2,3 it should always be strongly suspected in patients with known thyrotoxicosis who have evidence of systemic decompensation, and in patients displaying signs and symptoms of thyrotoxicosis without a previous diagnosis of this disorder.4
Common features of thyroid storm
Central nervous system: anxiety, confusion, delirium, generalized tremors, coma
Cardiovascular: tachyarrhythmia (most commonly atrial fibrillation), Means-Lerman scratch (a murmur produced by a hyperdynamic pericardium rubbing against the pleura),5 congestive heart failure, cardiac shock
Gastrointestinal: nausea, vomiting, diarrhea, abdominal pain
Respiratory: dyspnea, tachypnea
Others: fever, hyperhidrosis, skin hyperemia.
Scoring systems to distinguish thyrotoxicosis from thyroid storm, such as the Burch-Wartofsky Point Scale6,7 and the Japan Thyroid Association Criteria,8 are widely available in apps and online. They are sensitive but lack specificity and remain unvalidated.9
The Burch-Wartofsky Scale awards points for fever, central nervous system effects, gastrointestinal and hepatic dysfunction, tachycardia, heart failure, and atrial fibrillation, and for any precipitating event. A score of 45 or higher is considered highly consistent with thyroid storm, 25 through 44 suggests an impending storm, and less than 25 makes a diagnosis of thyroid storm unlikely.6,10 If we enter the data for our patient, her Burch-Wartofsky score was 65 and was therefore highly consistent with thyroid storm.
LABORATORY FINDINGS IN HYPERTHYROIDISM
2. Which one of the following would further support the diagnosis of hyperthyroidism?
Low uptake on radioactive iodine uptake scanning
Thyroperoxidase antibodies in the serum
Elevated reverse triiodothyronine (T3) level
A low thyroid-stimulating hormone (TSH) level and high free thyroxine (T4) level
Radioactive iodine uptake can be high or low in cases of thyroid storm. High uptake may represent rapid thyroidal turnover of iodine.2 On the other hand, iodine uptake may be low in patients experiencing thyroid storm who previously had thyroiditis, had received exogenous thyroid hormone, had been exposed to intravenous contrast, or had used amiodarone.
Thyroperoxidase antibodies in the serum suggest underlying autoimmune thyroid disease. While most commonly found in patients with Hashimoto thyroiditis, they may also be present in Graves disease. Their titers correlate with risk of progression to overt hypothyroidism but do not reflect thyroid function or thyroid storm.
Reverse T3 is metabolically inactive and has limited clinical value for routine testing of thyroid function.
Low TSH and high free T4 levels. A low (suppressed) TSH level (< 0.05 mIU/L) combined with elevated free T4 (> 1.6 ng/dL) support the diagnosis of thyrotoxicosis. However, no T3 or T4 cutoff level exists for diagnosing thyroid storm.
Though scoring systems to diagnose thyroid storm are available, ultimately the diagnosis is based on the overall clinical picture and the physician’s judgment. Levels of thyroid hormones during thyroid storm may be in some cases similar to those in a person with stable hyperthyroidism, and therefore, T3 and T4 levels are not reliable as diagnostic criteria.6,11
TSH levels, on the other hand, do have acceptable sensitivity and specificity to assess overall thyroid function, if pituitary function is normal.12
Other, nonspecific laboratory findings during thyroid storm may include mild hyperglycemia (due to inhibition of insulin release and increased glycogenolysis caused by catecholamines), mild hypercalcemia (secondary to increased bone resorption), elevated aminotransferase and alkaline phosphatase levels (related to liver dysfunction or from increased bone turnover), and leukocytosis or, conversely, leukopenia.9
CAUSES AND TRIGGERS OF THYROID STORM
3. Which of the following most likely placed this patient at risk of thyroid storm?
Vancomycin
Liver transplant
Absence of preexisting thyroid illness
Contrast-enhanced CT
Systemic infection
Underlying causes of thyrotoxicosis
Primary hyperthyroidism: Graves disease, toxic multinodular goiter, toxic adenoma, functioning thyroid carcinoma metastases, activating mutation of the TSH receptor, struma ovarii
Secondary hyperthyroidism: TSH-secreting pituitary adenoma, chorionic gonadotropin-secreting tumors, gestational thyrotoxicosis
Thyrotoxicosis without hyperthyroidism: subacute thyroiditis, silent thyroiditis including postpartum thyroiditis, ingestion of excess thyroid hormone (thyrotoxicosis factitia), and other causes of thyroid destruction such as amiodarone, radiation, and adenoma infarction.
Precipitants of thyroid storm
Surgery: thyroid surgery (“surgical storm”), non-thyroid surgery, manipulation of the thyroid gland
Cerebrovascular causes: myocardial infarction, venous thromboembolism, cerebrovascular disease
Neoplasms: struma ovarii, metastatic thyroid cancer
Endocrine diseases: Graves disease, thyroiditis, multinodular goiter, solitary toxic adenoma, diabetic ketoacidosis, hypoglycemia
Drugs: interferon, amiodarone, abrupt cessation of thionamide therapy (rare), interleukin 2 therapy, anesthetics, salicylates, pseudoephedrine
Others: systemic infections, thyroiditis, pregnancy, parturition, trauma, burns, radiocontrast dye, emotional stress
No known precipitant in many patients.13
The most likely risk factor in our patient
Considering the many possible causes of thyrotoxicosis and risk factors for thyroid storm, which was the most likely risk factor in our patient?
Vancomycin and liver transplant per se are not known risk factors for thyroid storm, but some anesthetic drugs used during surgical procedures are known triggers. The etiology of the patient’s liver disease and the medications used to treat it should be carefully reviewed, as medications used to treat viral hepatitis such as interferon alfa or interleukin 2 have been associated with thyrotoxicosis.14
Absence of preexisting thyroidal illness does not increase the risk of thyroid storm, but it also does not preclude it. Although thyroid storm usually occurs in the setting of hyperthyroidism such as Graves disease, it can happen in normothyroid patients. However, a precipitating event such as surgery, infection, myocardial infarction, cerebrovascular events, or exposure to iodinated contrast dye is typically needed to jump-start the process.2,6,15
Contrast-enhanced CT. Exogenous iodine, as in CT contrast media, should suppress synthesis and release of thyroid hormone (the Wolff-Chaikoff effect). But this effect is only temporary, and within a few days to weeks hyperthyroidism can develop (the Jod-Basedow phenomenon), particularly in patients with subclinical multinodular goiter or Graves disease.16
Infection can lead to an increase in cytokines including tumor necrosis factor alpha, interleukin 1, and interleukin 6 as part of an inflammatory response. This results in increased expression of proteins involved in thyroid hormone metabolism and transport and also of cell receptors, ultimately triggering thyroid storm.
Therefore, receiving multiple doses of iodinated radiocontrast dye and systemic infection were the likely precipitants of thyroid storm in our patient.
INITIAL TREATMENT
4. Which one of the following is the most appropriate next step in this patient’s treatment?
A beta-blocker and a thionamide
An iodide solution
Anticoagulation
Aspirin
An iodide solution would not be appropriate, as it can exacerbate hyperthyroidism unless a thionamide is given at least 1 hour beforehand to block iodine organification (incorporation into thyroglobulin) and resultant new thyroid hormone synthesis.2
Anticoagulation would also be inappropriate, unless thyroid storm were precipitated by pulmonary embolism or myocardial infarction, or if the patient develops atrial fibrillation. Petersen and Hansen17 found that atrial fibrillation is common in thyrotoxicosis, but the risk of stroke was not higher in patients with thyrotoxicosis with atrial fibrillation than in those with thyrotoxicosis without atrial fibrillation. Thus, the decision to start anticoagulation should be guided by the same risk-stratification criteria as in a patient without thyroid storm.
Aspirin is not recommended, owing to the possibility of it decreasing protein binding and thus increasing levels of free active thyroid hormone.2 Acetaminophen is preferred if antipyretic therapy is required.
A beta-blocker, propylthiouracil (a thionamide), and glucocorticoids were started in our patient. These medications, along with supportive care, led to improvement in her symptoms and cardiac function.
Optimal treatment of thyroid storm
Patients with thyroid storm are critically ill and have a high mortality risk.18 Therefore, treatment and resuscitative measures should begin as early as possible.
Management of thyroid storm involves the same principles that apply to uncomplicated hyperthyroidism, but additional medications and higher and more frequent dosing are often required.9
Optimal treatment of thyroid storm has the following 5 main goals6:
Reduce thyroid hormone synthesis and secretion
Block thyroid hormone actions at the cellular level
Reverse systemic decompensation (eg, hyperthermia, dehydration, congestive heart failure, arrhythmia)
Treat the precipitating event
Establish long-term therapy.
These goals can be achieved with a regimen commonly consisting of multiple medications with different mechanisms of action, as described below.
Thionamides
Both propylthiouracil and methimazole effectively inhibit hormone synthesis and can be used to treat thyroid storm.2 However, propylthiouracil has the added benefit of decreasing peripheral T4-to-T3 conversion in a dose-dependent fashion.19,20 Since T3 is the active form of thyroid hormone, propylthiouracil is in theory superior to methimazole in treating thyroid storm.18,20
Once patients are clinically stable, their propylthiouracil can be changed to methimazole, which requires less frequent dosing and has a lower risk of hepatotoxicity.21 Methimazole should be avoided in the first trimester of pregnancy as it has been found to cause birth defects.22
Exogenous iodine
Exogenous iodine decreases the release of preformed hormone, but new hormone synthesis must first be blocked with a thionamide, as underlying thyroid pathology may otherwise result in increased T3 and T4 production. Additionally, the thyroid iodide transport system adapts to increased levels of iodine and eventually escapes inhibition within 2 weeks,2,9 which may exacerbate thyrotoxicosis.23 Also, inhibiting the thyroid gland with exogenous iodine may delay the patient’s treatment with radioactive iodine.2,6
Glucocorticoids
Glucocorticoids decrease peripheral conversion of T4 to T3, and ameliorate the partial adrenal insufficiency commonly seen during thyroid storm that is due to excessive metabolic degradation of corticosteroids and that increases the risk for acute cortisol deficiency because of increased cortisol turnover and diminished reserves.24
Beta-blockers
Beta-blockers are a cornerstone in the management of thyroid storm, as they blunt the associated adrenergic surge, ie, a sudden and dramatic increase in catecholamines leading to severe increases in blood pressure and heart rate. Dose requirements may be high as a result of increased drug metabolism from hyperthyroidism.6
The most commonly used beta-blocker is propranolol, a nonselective drug that also decreases peripheral T4-to-T3 conversion, which usually requires a daily dose of 240 to 480 mg.3,25 Cardioselective beta-blockers such as atenolol or metoprolol can be considered, especially if the patient has relative contraindications to nonselective beta-blockers such as asthma or chronic obstructive pulmonary disease. In patients with decompensated heart failure, esmolol may be preferable, as it has a better safety profile in this population due to its very short half-life (9 minutes), so that any adverse effects may be quickly reversed.26,27
Of note: controlling tachycardia in thyroid storm may improve heart failure. Dosing of beta-blockers should be titrated to the desired effect and guided by the patient’s clinical condition.
In patients with absolute contraindications to beta-blockers, a nondihydropyridine calcium channel blocker such as diltiazem may control the heart rate.28
Bile acid sequestrants
Thyroid hormones are metabolized in the liver, where they are first conjugated with glucuronide and sulfate, then excreted in the bile into the intestine, and finally reabsorbed through the portal system. Therefore, bile acid sequestrants such as cholestyramine have been found to reduce thyroid hormone levels in patients with thyrotoxicosis by interfering with enterohepatic circulation and recycling of thyroid hormone.29–31
Plasmapheresis and surgery
In refractory cases, plasmapheresis or emergency surgery may be needed.32,33 Plasmapheresis removes thyroid hormones, catecholamines, autoantibodies (in the case of Graves disease), and cytokines that trigger inflammation, all of which are undesirable in thyroid storm.32
If surgery is determined to be the best approach and the patient is receiving an iodide solution (eg, saturated solution of potassium iodide, Lugol solution), the surgery should be done within 8 to 10 days to avoid the Jod-Basedow phenomenon.2,9 If an iodine solution is not used, there is no additional increased risk in deferring surgery. Surgery in patients with elevated thyroid hormones, however, has extraordinary cardiovascular risks such as ischemic heart disease, atrial fibrillation, and congestive cardiac failure that require careful attention from the anesthesiology team.
Supportive care
Supportive care may include one or a combination of the following:
Antipyretics. Distress from pyrexia may be relieved with acetaminophen, which is preferred over salicylates, which affect protein binding and may increase the level of free thyroid hormone.2 Peripheral cooling with ice packs and cooling blankets can also be implemented.
Volume resuscitation. Dehydration is often due to insensible fluid loss, diarrhea, and vomiting.19 Also, several factors, including increased production of metabolic end products in a hypermetabolic state and direct stimulation of potassium channels in arterial smooth muscles by thyroid hormones, favor a state of general vasodilation.34 Volume management and electrolyte replacement are appropriate based on the patient’s condition and fluid status, keeping in mind that overenthusiastic administration of fluids could worsen heart failure.
Sedatives are used to manage delirium and agitation.
FURTHER CARE
5. After the patient is clinically stable, which one of the following would be the most appropriate next step in her management?
Continue propylthiouracil indefinitely
Thyroidectomy
Discontinue propylthiouracil, start methimazole, and evaluate for preexisting thyroid pathology such as Graves disease
Radioactive iodine treatment
Surgical consultation for thyroidectomy, antithyroid drugs, and radioactive iodine treatment should all be considered for definitive therapy if the patient is found to have underlying thyroid disease such as Graves disease.4 However, in our patient’s case, stabilizing her condition is the priority, while long-term therapy can be formulated later.
Stopping propylthiouracil, starting methimazole
Once a patient with thyrotoxicosis is in stable condition, propylthiouracil should be replaced by methimazole, which has a better safety profile. Common side effects of both medications include pruritus, rash, urticaria, arthritis, fever, nausea, and vomiting. More serious side effects include agranulocytosis, antineutrophil cytoplasmic antibody-positive vasculitis, and hepatotoxicity, all of which are more frequent with propylthiouracil. Additionally, hepatotoxicity due to propylthiouracil use is associated with hepatocellular inflammation and necrosis, likely explaining higher rates of liver failure with this drug compared with methimazole, which is associated with cholestatic dysfunction.35
Propylthiouracil should be discontinued at any time if aminotransferase levels reach more than 3 times the upper limit of normal, or if elevated levels at the onset of therapy increase further.4 These levels should then be monitored weekly until they return to normal.
Stopping other drugs
Glucocorticoids and iodine therapy should be discontinued once the patient’s condition is stable. Beta-blockers should be tapered and discontinued when thyroid function studies return to normal. Thionamides need to be titrated to maintain a euthyroid state, usually over weeks to months.20
Thyroid storm mortality rates have been decreasing in recent years, partly due to advances in treatment and earlier recognition of this medical emergency.36 However, even when death is prevented, significant morbidity in the form of end-organ damage may lead to long-term complications.
CASE CONCLUSION
Our patient’s condition stabilized with medical treatment. She underwent a workup for underlying thyroid disease after discharge from the hospital, but none was found. Likely precipitants of her thyroid storm were repeated exposure to intravenous iodine-containing contrast and systemic infection.
TAKE-HOME POINTS
Thyroid storm requires a high level of suspicion (particularly in patients with preexisting thyroid disease), prompt recognition, and intensive medical therapy, in view of its high mortality rate. Its symptoms are not specific.
Most cases of thyroid storm happen in the setting of underlying Graves disease; however, it may also occur in patients with normal thyroid function if they are exposed to the right triggers.
TSH is the best single test to evaluate thyroid function.
Hormone levels in patients experiencing thyroid storm are comparable to those in patients with stable thyrotoxicosis. Therefore, no cutoff values for the diagnosis exist.
Management of thyroid storm is complex, based on the specifics of each case, and usually involves multiple treatments.
DISCLOSURES
Dr. Stancampiano has disclosed work as principal or co-investigator of funded research for Gilead Sciences. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.