ABSTRACT
Cirrhosis has been regarded as a hypocoagulable state associated with an increased risk of bleeding. But patients with cirrhosis also have a high incidence of thrombotic complications, challenging this dogma. We now recognize that in cirrhosis there is a simultaneous decrease in both clotting and anticlotting factors, leading to a new equilibrium. Conventional coagulation tests such as the platelet count and prothrombin time do not assess the reduced anticoagulation factors in cirrhosis and overestimate the bleeding risk, and any intervention based on these test results can lead to thrombotic complications. This article reviews the changes in hemostasis associated with cirrhosis, newer tests for assessing coagulation, and preprocedural minimization of coagulopathy.
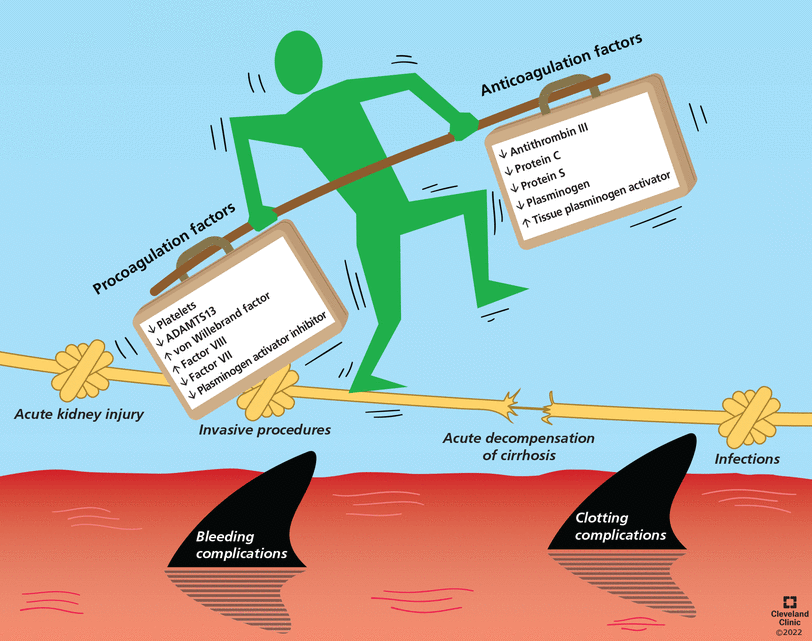
The rebalanced hemostasis of cirrhosis is a delicate equilibrium of antithrombotic and prothrombotic changes associated with decreased synthetic liver function, inflammation, and endothelial and platelet activation related to cirrhosis.
There is no evidence to support routine transfusion of blood products to "correct" coagulopathy before low-risk procedures, since this does not decrease procedure-specific bleeding risk and is itself associated with significant risk.
Viscoelastic tests such as thromboelastography may better reflect the true state of cirrhotic hemostasis, but further studies are needed to establish validated transfusion thresholds.
Coagulopathy—characterized by prolonged prothrombin time, elevated international normalized ratio (INR) of the prothrombin time, prolonged activated partial thromboplastin time, low fibrinogen levels, and low platelet counts—is a hallmark of advanced cirrhosis. Traditionally, cirrhosis has been considered a hypocoagulable state in which the risk of life-threatening bleeding complications is increased.1–3
Evidence of this comes from the PRO-LIVER study,4 which prospectively followed 280 patients with cirrhosis for a median of 1,129 days. Significant bleeding events occurred in 5.45% of patients per year.4 The bleeding rate is higher in patients with advanced liver disease who need to be admitted to the hospital because of acute decompensation of their liver disease, or for patients with cirrhosis who need to be admitted to the intensive care unit for any reason.3,5 Most of these bleeding events are gastrointestinal and most are thought to be related to elevated portal pressures.4,5 Importantly, markers of coagulopathy such as elevated INR, thrombocytopenia, and low fibrinogen levels have not been shown to correlate with or predict the risk of bleeding events accurately.6
However, patients with cirrhosis also have a high incidence of thrombotic complications such as portal vein thrombosis and venous thromboembolism, which are independently associated with significant morbidity, acute hepatic decompensation, and death.7,8 The incidence of portal vein thrombosis in patients with cirrhosis has varied widely in different studies, owing to differences in the populations studied, but it is higher than in patients without cirrhosis.9,10 A rate ranging between 3.2% and 4.1% at 1 year after diagnosis is often cited and increases over time.11,12
In a large case-control study, the relative risk of venous thromboembolism in patients with cirrhosis was found to be 1.74 (95% confidence interval [CI] 1.54–1.95) compared with patients without liver disease.13 These findings were echoed by data from the Multiple Environmental and Genetic Assessment study,14 which showed that in hospitalized patients, liver disease was associated with significantly increased risk of venous thromboembolism (adjusted odds ratio [OR] 1.7, 95% CI 1.0–2.9).14
This increased risk of thrombotic events challenges the notion that patients with cirrhosis are “autoanticoagulated” and highlights the need for a more nuanced evaluation of the coagulopathy of cirrhosis.
In fact, the liver synthesizes most proteins of the coagulation system. Cirrhosis results in a simultaneous decrease in both procoagulant and anticoagulant factors, resulting in a delicate state of rebalanced hemostasis,15 metaphorically illustrated in Figure 1. This coagulation profile is unique to the individual patient and is influenced by the etiology of liver disease, disease severity, acute illnesses, and ongoing therapy.16,17 Conventional coagulation tests such as prothrombin time, INR, and platelet count measure procoagulant factors but not the anticoagulation factors, and therefore they are inadequate to accurately assess bleeding risk and guide management.2,11
Coagulation and anticoagulation in patients with cirrhosis are rebalanced due to simultaneous decreases in clotting and anticlotting pathways. However, this balance is dynamic, and concomitant conditions such as infection and acute kidney injury can tip the balance, resulting in a clotting or bleeding complication. (ADAMTS13 = a disintegrin and metalloproteinase with thrombospondin type 1 motifs, member 13)
The purpose of this review is to elucidate the current understanding of coagulopathy of cirrhosis, how to assess it, and how to manage bleeding risk in patients about to undergo invasive procedures.
PATHOPHYSIOLOGY OF HEMOSTASIS
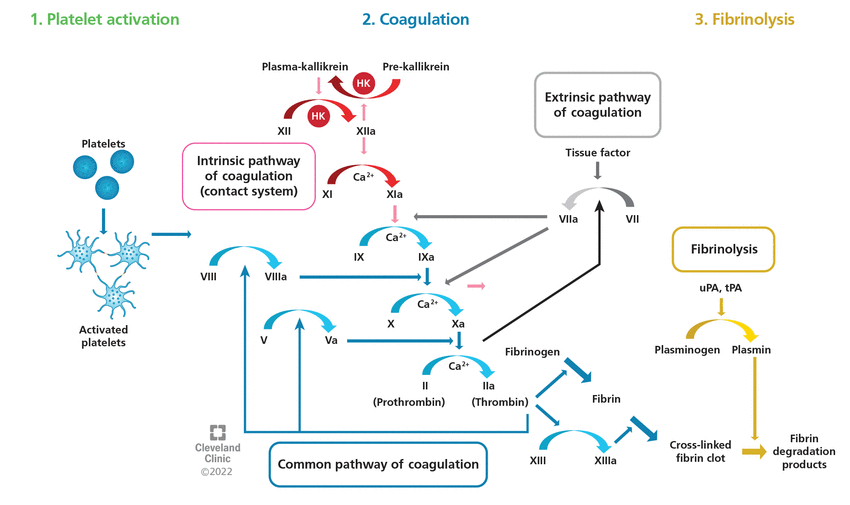
The normal hemostatic process comprises 3 phases (Figure 2):
Platelet activation: When the vessel wall is injured, subendothelial collagen and tissue factor are exposed, triggering platelet activation and primary hemostasis with adhesion of the initial platelet plug through interactions with von Willebrand factor, factor VIII, glycoprotein IIb/IIIa receptors, and fibrinogen.
Coagulation: Sequential activation of prothrombotic coagulation factors leads to thrombin activation, thrombus formation, and thrombus stabilization through conversion of fibrinogen to fibrin and cross-linking of fibrin polymers.
Fibrinolysis or clot dissolution.
The 3 phases of coagulation, involving a range of clotting factors (as Roman numerals).
HK = high-molecular-weight kininogen; tPA = tissue plasminogen activator; uPA = urokinase plasminogen activator
Cirrhosis affects all 3 phases, leading to a delicate new equilibrium—the rebalanced hemostasis of cirrhosis. This new balance is easily disturbed and tipped toward either bleeding or thrombosis by acute events such as infection, renal failure, and invasive procedures with or without prophylactic transfusions.
A new balance in platelet activation
Fewer platelets
Thrombocytopenia is common in cirrhosis and portal hypertension, likely due to increased platelet destruction, reduced hepatic synthesis of thrombopoietin, and increased splenic aggregation (which further increases thrombopoietin clearance).18 Tripodi et al19 found that platelet counts as low as 60 × 109/L in plasma from patients with cirrhosis were still sufficient to yield in vitro thrombin generation similar to that in plasma from healthy controls with normal platelet counts. This highlights the importance of qualitative alterations in platelet activation beyond the quantitative decrease in platelet counts in cirrhosis.
More von Willebrand factor
In contrast to the coagulation factors synthesized by the liver, von Willebrand factor is produced, stored, and released by the vascular endothelium. Its levels are preserved or even increased in cirrhosis.20 Levels of von Wille-brand factor have also been shown to progressively increase with more advanced liver disease. Lisman et al20 reported that, compared with healthy controls, patients with Child-Pugh class A cirrhosis had von Willebrand factor antigen levels 380% higher, those with class B cirrhosis had levels 500% higher, and those with class C cirrhosis had levels 760% higher. Similarly, von Willebrand factor levels were 790% higher in patients with acute liver failure.20
Less ADAMTS13
ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motifs, member 13) is a potent inhibitor of von Willebrand factor and is reduced in cirrhosis.21 However, data are conflicting as to the magnitude of this decrease and its correlation with disease severity, perhaps partly because ADAMTS13 is difficult to measure and results vary with different assays.19
Thus, the data suggest that in patients with cirrhosis, thrombocytopenia is countered by a simultaneous increase in von Willebrand factor and a decrease in ADAMTS13, resulting in only mildly decreased or even increased platelet activity. Indeed, the excess risk of thrombosis observed in patients with biliary cirrhosis has been attributed to platelet activation.22
Rebalanced coagulation vs anticoagulation
The coagulation cascade is driven by procoagulant factors and inhibited by anticoagulant factors.23,24 As both types of factors are predominantly produced in the liver, this phase is rebalanced in cirrhosis.
Procoagulation factors are decreased
Release of tissue factor from the endothelium activates factor VII, forming the tissue factor-VIIa complex, which leads to activation of factors V, IX, and X and ultimately to conversion of prothrombin to thrombin. As all coagulation factors except for factor VIII (produced in hepatic sinusoidal endothelial cells) are synthesized in hepatocytes, conventional coagulation tests that assess procoagulant factors tend to suggest a hypocoagulable state in conditions of hepatic synthetic dysfunction such as cirrhosis or liver failure: eg, the prothrombin time will be prolonged and the INR will be high.
Anticoagulation factors are also decreased
Anti-coagulation factors, in contrast, exert their effect in the endothelium and are difficult to quantify in vitro.25 For example, tissue factor pathway inhibitor forms a complex with activated factor X. It inhibits the tissue factor-factor VIIa complex and facilitates degradation of factors V and VIIIa. Tissue factor pathway inhibitor has been shown to be reduced in cirrhosis.
Moreover, the key anticoagulant proteins activated protein C and protein S have been similarly shown to be reduced in cirrhosis. Activated protein C with protein S as a cofactor inhibits activated factors V and VII and thus thrombin formation. The activity of protein C is regulated in the endothelium by thrombomodulin, and “thrombomodulin resistance” has been demonstrated in plasma from patients with cirrhosis.26,27 This affirms the hypercoagulable effect of decreased hepatic synthesis of proteins C and S in vitro.
In fact, the thrombin generation potential in plasma from cirrhotic patients has been demonstrated to be similar to that of noncirrhotic patients, confirming the rebalanced state of hemostasis in cirrhosis.19
Decreased fibrinolysis
Plasmin-mediated fibrinolysis and clot dissolution is the final step in hemostasis. Plasmin is activated from plasminogen by fibrin, as well as by tissue plasminogen activator, urokinase plasminogen activator, and activated factor XII. Conversion of plasminogen to plasmin is inhibited by thrombin activatable fibrinolysis inhibitor and plasminogen activator inhibitor.15,28 A decrease in the plasmin activation pathway results in a hypofibrinolytic state, while an increase results in a hyperfibrinolytic state.
Cirrhosis has been shown to be associated with both quantitative and qualitative changes in the fibrinolytic pathway. Plasminogen levels are reduced in patients with cirrhosis, likely due to the combined effects of decreased production and increased consumption related to the frequent activation of the coagulation cascade from ongoing inflammation.24 Oxidative stress, leading to modifications of fibrin, and increased sialic acid content and altered calcium binding lead to decreased clot permeability and impaired fibrinolysis.29,30 Together these qualitative and quantitative changes result in a net decrease in fibrinolysis in cirrhosis.
Inflammation and infection can tip the balance
Systemic inflammation is an important factor in the development and progression of chronic liver disease, acute liver failure, and acute-on-chronic liver failure. Moreover, patients with liver disease are at increased risk of both primary and secondary infections, which in turn contribute to disease progression.31–33
Several mechanisms link inflammation and coagulopathy. Inflammatory cytokines lead to direct activation of platelets and the endothelium, and endothelial activation in turn prompts the release of tissue factor and von Willebrand factor. Tissue factor activates the extrinsic coagulation cascade, and increasing levels of von Willebrand factor further promote platelet activation and adhesion. Increased levels of fibrinogen, an acute-phase reactant, can tip the balance toward a more hypercoagulable state. Similarly, inflammation-induced expression of plasminogen activator inhibitor 1 further inhibits fibrinolysis. Eventually, prolonged activation of a systemic inflammatory response can result in exhaustion of thrombotic and thrombolytic systems, leading to a state of consumptive coagulopathy.34
These mechanisms highlight the complexity of coagulopathy of advanced liver disease and emphasize the importance of individualized assessment and management of coagulopathy in patients with cirrhosis and liver failure, particularly in patients with systemic inflammation or sepsis, or both.
ASSESSING CLOTTING AND ANTICLOTTING IN CIRRHOSIS
An accurate assessment of the coagulation system is paramount in the clinical management of cirrhosis. An ideal test should evaluate both the clotting and the anticlotting pathways to provide an accurate assessment of hemostasis to guide therapy.
Conventional tests assess only clotting and may overestimate bleeding risk
The conventional tests for assessing coagulation are the prothrombin time, INR, platelet count, and fibrinogen level. These tests cannot assess the impact of the anticoagulant mechanisms outlined above2 and may overestimate the bleeding risk in cirrhosis. Prolongation of the prothrombin time and activated partial thromboplastin time indicates a decrease in hepatic synthesis of procoagulation factors and correlates with hepatic function, but this does not adequately quantify bleeding risk.
Of importance is that the INR is standardized and validated using plasma from patients receiving vitamin K antagonists such as warfarin. There is no standard reference plasma that could be used in clinical practice to express a normalized ratio of the prothrombin time for patients with cirrhosis.15
Similarly, the quantitative decrease of platelet counts and fibrinogen levels in cirrhosis is balanced by qualitative changes in platelet activation and fibrinolysis, making the absolute values difficult to interpret in the context of the rebalanced state of hemostasis.
Viscoelastic tests are promising but need more study
Viscoelastic tests such as thromboelastography and rotational thromboelastometry provide a holistic evaluation of the coagulation process, assessing clot formation, clot propagation, maximum clot strength, and fibrinolysis as a reflection of shear stress in vitro. Viscoelastic tests are performed using whole blood, assessing coagulation in a more global, functional, and potentially clinically relevant fashion than individual coagulation parameters.
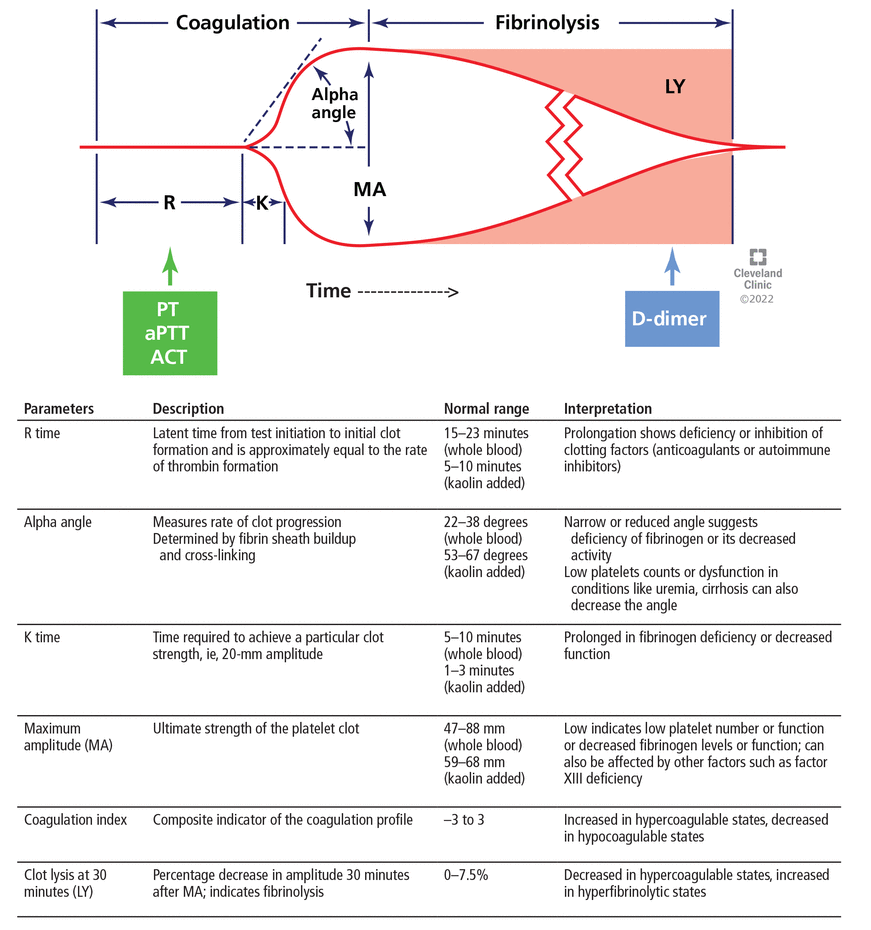
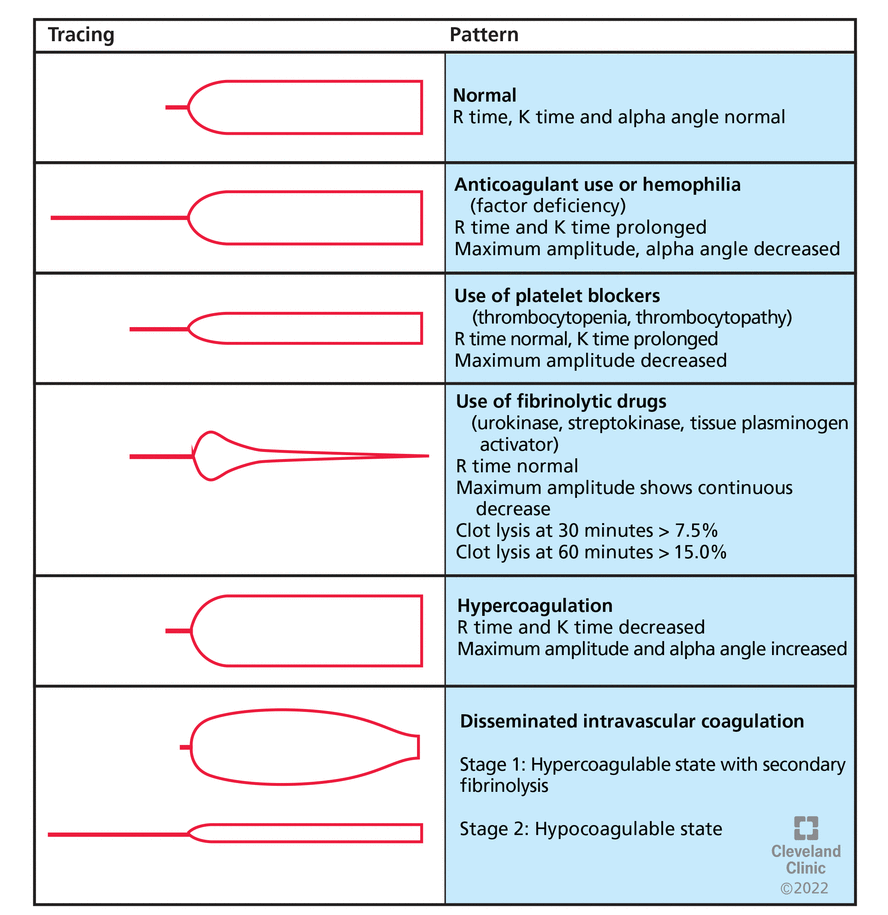
Thromboelastography is performed with a torsion pin suspended in an oscillating cup containing whole blood.35 As the blood begins to clot, the initial platelet clot and fibrin strands move this pin. The deflection of the pin is proportional to clot strength and is displayed graphically (Figure 3).36 The pattern is altered in patients with abnormal hemostasis (Figure 4).
Thromboelastography is a promising test of coagulation. The horizontal axis represents time, the vertical axis represents deflection of the thromboelastography probe. The R time is also assessed by tests such as the prothrombin time (PT), the activated partial thromboplastin time (aPTT), and the activated clotting time (ACT). D-dimer is used to assess fibrinolysis.
Adapted from Singh AD, Shalimar. Use of blood products and drugs before procedures in patients with cirrhosis. Clin Liver Dis (Hoboken) 2020; 16(4):153–157. doi:10.1002/cld.906, reference 36.
Results of thromboelastography in various conditions.
Rotational thromboelastometry uses a stationary cup, a rotating pin, and optical methods to measure clot formation instead of the shearing forces used by thromboelastography. It is considered less vulnerable to movement and vibration.37,38
Viscoelastic tests were developed as point-of-care tests to provide rapid results during surgery or in the trauma bay—in 15 to 20 minutes, compared with conventional tests, which require significantly longer turnaround times (eg, 45–60 minutes for prothrombin time and INR).
Viscoelastic testing is well established in trauma care and cardiothoracic surgery, where its use has significantly reduced blood product utilization and has led to improved outcomes.39 It has been shown to predict the need for massive transfusions during liver transplant, and transfusion protocols guided by viscoelastic testing during liver transplant surgery have been shown to reduce the intraoperative use of blood products without an associated increased rate of bleeding.40,41
Outside the operating room, a small study by Chau et al42 found that abnormal results on thromboelastography were associated with risk of rebleeding in patients with esophageal variceal hemorrhages, but data on predicting spontaneous bleeding risk remain limited.
Thromboelastography is highly reproducible and routinely shows normal coagulation profiles in patients with cirrhosis in stable condition.43 For example, in 273 patients with compensated cirrhosis, Stravitz44 found that the thromboelastography parameters were within the normal range, even though the prothrom-bin time and INR were prolonged. Similarly, only 14 (27%) of 51 patients with stable cirrhosis had abnormal clotting times on rotational thromboelastography in a study by Tripodi et al.45
However, the coagulopathy of liver disease is dynamic, and thromboelastography displays a more hypocoagulable profile with increasing severity of liver disease as well as in the setting of acute decompensation. De Pietri et al46 compared the coagulation profiles of 261 patients with decompensated cirrhosis and Model for End-Stage Liver Disease scores between 15 and 40 with those of 40 healthy participants. The latency time between test initiation and clot formation (R time) was prolonged in 41.5% of the patients, and the ultimate strength of the clot (maximal amplitude) was weaker in 79.3% patients with cirrhosis.46
Further, Lloyd-Donald et al47 reported that 34 critically ill patients with Child-Pugh class C cirrhosis had longer R times, weaker clot strength, and reduced clot lysis compared with 157 healthy controls. This further supports the dynamicity of liver disease and the impact of underlying cirrhosis on coagulability.
However, certain limitations restrict widespread clinical implementation of viscoelastic testing. The normal limits are not standardized, and clinical trials have used different cutoff values as indications for treatment.48–51 Moreover, the impact of concomitant conditions such as sepsis or acute kidney injury on these results has not been studied.51
Accordingly, the current guidelines from the American Association for the Study of Liver Diseases11 and a technical review from the American Gastroenterology Association52 state that though viscoelastic tests are promising, their clinical utility in predicting bleeding risk in patients with liver disease is yet to be firmly established. The current Society for Critical Care Medicine guidelines53 recommend the use of viscoelastic testing over the conventional tests in patients with cirrhosis in the intensive care unit. The clinical applicability of these tests is detailed in the next section.
PROPHYLACTIC OPTIMIZATION OF COAGULOPATHY
Coagulopathy in cirrhosis is widely interpreted as a risk factor for bleeding after an invasive procedure. The need to minimize the risk of coagulopathy before procedures is a common dilemma for practitioners.
The risk of bleeding is mainly determined by the type of procedure, the clinical scenario, comorbidities, use of ultrasonographic guidance, and operator experience.54 Various procedures are classified as high-, intermediate-, or low-risk (Table 1).2,55
Bleeding risk associated with invasive procedures
Traditional coagulation tests do not predict post-procedural bleeding complications.56 A meta-analysis of 29 studies including 13,276 patients found that neither elevated INR (OR 1.52, 95% CI 0.99–2.33) nor thrombocytopenia (OR 1.24, 95% CI 0.55–2.77) significantly increased the risk of bleeding in patients with cirrhosis.57 Moreover, the mean INR did not significantly differ between patients with bleeding complications and those without. However, there was significant heterogeneity (I2 = 51%) in the pooled results, likely attributable to differences in the severity of thrombocytopenia in various studies. The risk of bleeding was associated with the type of invasive procedure, but not with the results of conventional tests of coagulopathy.57
Paracentesis, the most commonly performed procedure in patients with cirrhosis, is considered low-risk and can be done safely even if the results of conventional coagulation tests are abnormal. In a study of 1,100 therapeutic paracenteses in 628 patients, of whom 513 had cirrhosis of the liver and in whom the mean INR was 1.7, no patients received prophylactic preprocedural correction of INR, and no significant bleeding events (defined as bleeding requiring hospitalization) were reported.58
By comparison, a study of 2,740 percutaneous liver biopsies reported an increased risk of bleeding in patients with INR greater than 1.3 and platelet counts less than 60 × 109/L.59 This area clearly needs further study.
Transfusion may not reduce bleeding, and it has its own risks
Furthermore, no studies have shown that giving prophylactic transfusions of fresh frozen plasma to correct an elevated INR reduces the risk of procedure-related bleeding in patients with cirrhosis. Also, in vitro experiments have demonstrated that transfusion of fresh frozen plasma does not increase coagulation potential in patients with cirrhosis, as it supplies both procoagulant and anticoagulant factors in equal amounts. As a result, the increase in plasma levels of procoagulant factors may correct an elevated INR, but thrombin-generating potential does not change, or may even decrease.60,61
The current standard of practice is prejudiced toward the hypocoagulable state of coagulopathy and disregards the risks associated with blood product transfusion.36 Acutely ill patients with cirrhosis are at increased risk for transfusion-related lung injury and complications from transfusion-related circulatory overload.62 In a small classic study, every 100 mL of volume expansion increased the portal pressure by 1.4 cm H2O (1.03 mm Hg).63 It is estimated that lowering the INR from 2.0 to 1.5 requires transfusion of 1.5 L, which would raise the portal pressure by approximately 15.5 mm Hg.64 This is important, since an elevated hepatic venous pressure gradient (> 12 mm Hg) is associated with an increased risk of variceal hemorrhage.65,66
Large-volume blood product transfusions aimed at correcting an elevated INR can therefore translate to increased bleeding complications. This is supported by a recent multicenter retrospective study, which found that transfusion of fresh frozen plasma to manage acute variceal bleeding increased the risk of death within 42 days (OR 9.41, 95% CI 3.71–23.90).67 Notably, patients who received fresh frozen plasma had a higher INR at baseline evaluation, and the patients who had died by 42 days had received a median of 3 units of fresh frozen plasma, compared with 0 units in those who were alive at 42 days.
Accordingly, the current recommendations advise against routine preprocedural correction of INR or thrombocytopenia in patients with cirrhosis, particularly for low-risk procedures.2,11,52,68
Can viscoelastic testing reduce transfusions?
As reviewed, viscoelastic tests may more accurately assess the global coagulation status. Recent randomized controlled trials have evaluated the impact of thromboelastography-guided prophylactic transfusion protocols compared with the standard of care for the use of blood products and bleeding complications for invasive procedures and in the setting of variceal and nonvariceal gastrointestinal bleeding.48–60 In all the studies, thromboelastography-guided therapy significantly reduced transfusion of blood products (fresh frozen plasma and platelets) compared with the standard of care, while the incidence of postprocedure-related bleeding between the groups was similar.
However, several limitations need to be considered when interpreting these findings. Most importantly, in these trials, the standard of care aimed to “correct” the INR and platelet counts to arbitrary near-normal thresholds. This is not in line with current restrictive recommendations for transfusion. Furthermore, transfusion thresholds in the thromboelastography-based protocols varied among trials, and there are currently no uniform and well-established transfusion thresholds for viscoelastic tests.49,50,69 It remains unclear if a restrictive transfusion strategy based on viscoelastic testing is superior to a restrictive strategy based on conventional tests. The small number of patients and the very low bleeding rates observed in these trials further limit their generalizability, as they may therefore be underpowered to detect true differences between the 2 strategies.
In sum, viscoelastic tests are promising tools to both assess the coagulopathy of cirrhosis and guide preprocedural management of hemostasis, but their current use is limited by a lack of validated transfusion thresholds and limited clinical availability outside of the operating room or research setting.2 Further large-scale studies are needed to establish such thresholds to facilitate translation into general clinical practice.
DISCLOSURES
Dr. Lindenmeyer has disclosed authorship for Merck Manuals. The other authors have disclosed no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.