Because the majority of patients with acute decompensated heart failure remain at high risk for in-hospital hypotension owing to low cardiac output and neurohormonal blockade from guideline-directed medical therapy,1 we recommend a tailored approach to risk-stratify patients with acute decompensated heart failure that focuses on avoidance, early recognition, and management of symptomatic and clinically significant hypotension.
HYPOTENSION
Blood pressure varies widely within the course of hospitalization for acute decompensated heart failure, and elevated systolic blood pressure (SBP) allows for easier initiation of guideline-directed medical therapy,2 whereas in-hospital hypotension is associated with unfavorable outcomes.1,2
Hypotension may be either absolute (eg, SBP less than 90 mm Hg or mean arterial pressure less than 65 mm Hg) or relative (eg, SBP drop more than 40 mm Hg) and becomes clinically relevant when persistent and associated with symptoms such as dyspnea, chest pain, syncope, headache, visual disturbances, emesis, or fatigue.2 It is commonplace for patients with heart failure to experience transient blood pressure drops shortly after medication dosing, but symptoms usually subside with heart failure improvement.2 Importantly, hypotension is not always a manifestation of shock, characterized by end-organ underperfusion. Hypotension may be either absolute (eg, SBP less than 90 mm Hg or mean arterial pressure less than 65 mm Hg) or relative (eg, SBP drop more than 40 mm Hg) and becomes clinically relevant when persistent and associated with symptoms such as dyspnea, chest pain, syncope, headache, visual disturbances, emesis, or fatigue.2 It is commonplace for patients with heart failure to experience transient blood pressure drops shortly after medication dosing, but symptoms usually subside with heart failure improvement.2 Importantly, hypotension is not always a manifestation of shock, characterized by end-organ underperfusion.
Factors that contribute to in-hospital hypotension
Numerous factors contribute to in-hospital hypotension in acute decompensated heart failure.1,2 Lower effective circulating volume caused by diuretic use and third-spacing is a key precipitating element. Arrhythmias, which can either induce systolic dysfunction or exacerbate underlying cardiomyopathies, commonly present with acute decompensated heart failure. Impaired vasoreactivity due to comorbid conditions (eg, diabetes or amyloidosis) may amplify the heart failure-induced vasodilatory state.1 Finally, hypotension may be a reflection of advanced pump failure resulting in inability to generate enough pressure to overcome the increased ventricular afterload and preload resulting from neurohormonal feedbacks (including sympathetic and renin-angiotensin system activation, as well as release of antidiuretic hormone).
Development of in-hospital hypotension in acute decompensated heart failure can limit the use of lifesaving therapies and lead to malperfusion with consequent end-organ damage.1,2 This is clinically relevant in patients with acute decompensated heart failure, where hypotension-induced kidney injury may prevent effective diuresis and require escalation to renal replacement therapy, thereby contributing to poor outcomes.3
Despite these factors, clinicians may accept in-hospital hypotension as a compromise to rapidly titrate guideline-directed medical therapy.1 In fact, the STRONG-HF trial (Safety, Tolerability, and Efficacy of Rapid Optimization, Helped by N-Terminal pro-B-type Natriuretic Peptide Testing, of Heart Failure Therapies) met the composite primary outcome of reduced risk of all-cause death or heart failure readmission at 6-month follow-up, driven by reduction in the latter.4 Therefore, early detection and correction of in-hospital hypotension is critical to mitigate patient risk and maximize benefits of guideline-directed medical therapy.
GUIDELINE-DIRECTED MEDICAL THERAPY: REAL-WORLD EXPERIENCE
Guideline-directed medical therapy underutilization is common for several reasons, including highly selected trial populations and the following5:
Enterprise-level factors (restrictive pharmacotherapy policy, inadequate health information technology, inaccessible multidisciplinary care)
Physician-level factors (knowledge or communication gaps, uncertainty about trial generalizability, concerns about contraindications, biased decision-making, clinical inertia)
Patient-level factors (preference against changing therapies, suboptimal health literacy or adherence, lack of affordability, side effects, comorbidities).5
Importantly, acute decompensated heart failure complicated by cardiogenic shock, acute coronary syndrome, or worsening kidney function is common in registries, but patients with these scenarios were excluded from inpatient initiation trials.1 Regardless, even trial-eligible patients remain undertreated.5 Few multifold strategies to increase guideline-directed medical therapy utilization have been tested in randomized controlled trials, and even fewer were successful.5
Guideline-directed medical therapy and hypotension
Hypotension is a recognized adverse effect and reason for withdrawal of treatment among landmark trials.1,5 Despite being a central safety criterion, it is important to note the heterogeneity of definitions, exclusion criteria, and incidence of adverse effects (Table 1).6–23 Actually, lowering blood pressure is not always bad. Patients enrolled in the EMPHASIS (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure)21 and PARADIGM-HF (Prospective Comparison of ARNi with ACEi to Determine Impact on Global Mortality and Morbidity in Heart Failure)17 had lower mortality risk (all-cause and cardiovascular causes in both studies) and reduced risk of hospitalization even with greater blood pressure reduction after guideline-directed medical therapy. This may suggest that short-term blood pressure-lowering effects of guideline-directed medical therapy are a tolerable trade-off for the long-term beneficial neurohormonal blockade.
Hypotension in landmark randomized controlled trials of guideline-directed medical therapy
Angiotensin-receptor–neprilysin (ARN) inhibitors and carvedilol were studied in patients with acute decompensated heart failure and SBP greater than 100 mm Hg and greater than 85 mm Hg, respectively.24 Patients with low SBP were more likely to discontinue therapy or have symptomatic hypotension. In contrast, stable patients with SBP greater than 100 mm Hg did not experience significant hypotension with sodium-glucose cotransporter 2 (SGLT-2) inhibitors.25 Mineralocorticoid receptor antagonist trials did not have blood pressure exclusion criteria, and even patients with SBP less than 105 mm Hg had positive safety end points.24
HYPOTENSION IN ACUTE DECOMPENSATED HEART FAILURE: A PROPOSED APPROACH
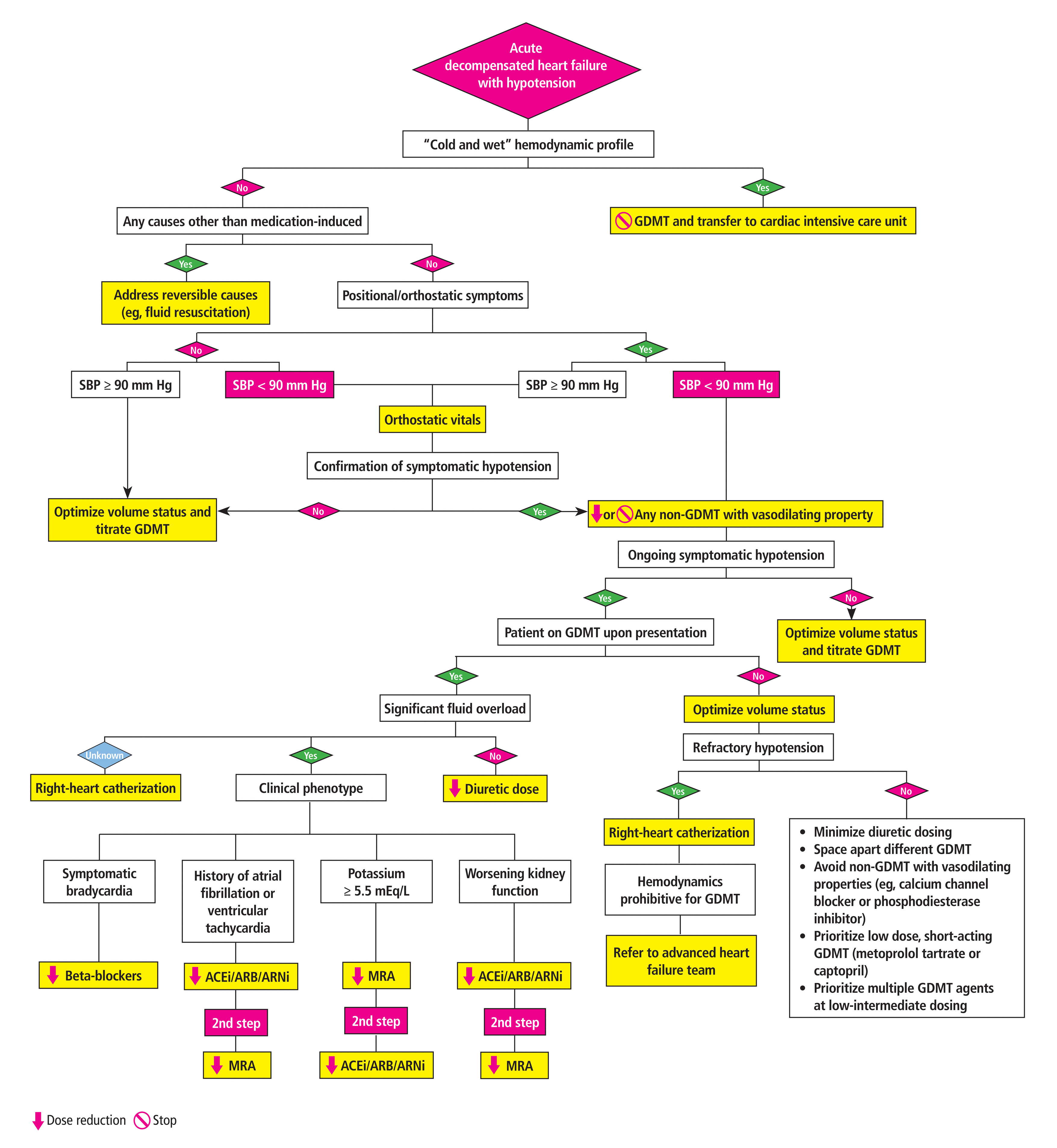
We risk-stratify patients with acute decompensated heart failure and focus on avoidance, early recognition, and management of symptomatic and clinically significant hypotension (Figure 1). Initially, clinicians should proactively screen for signs of impending circulatory shock that would require immediate escalation of care. A cardiology consultation would be appropriate to guide judicious guideline-directed medical management in patients with subtle signs of early compensated shock, including restlessness, pale and clammy skin, nausea and vomiting, tachycardia, tachypnea, delayed capillary refill, and narrow pulse pressure. As the initial compensatory mechanisms start failing, physical (eg, obtundation, oliguria, cold extremities, peripheral cyanosis) and laboratory (eg, hypoxia, lactic acidosis, renal dysfunction, or liver injury) signs of critical hypoperfusion may become apparent, and patients should be readily transferred to an intensive care unit.
Management algorithm for in-hospital hypotension in patients with acute decompensated heart failure.
ACEi = angiotensin-converting–enzyme inhibitor; ARB = angiotensin receptor blocker; ARNi = angiotensin-receptor–neprilysin inhibitor; GDMT = guideline-directed medical therapy; MRA = mineralocorticoid receptor antagonist; SBP = systolic blood pressure
Asymptomatic hypotension
The approach to hypotension in patients with acute decompensated heart failure needs to be tailored to patient-specific factors. For example, an SBP of 90 mm Hg would not be disproportionately low in a patient with a 10% ejection fraction in the absence of signs of hypoperfusion, whereas an SBP of 130 mm Hg may represent a relative hypertensive urgency. Alternative causes of hypotension (eg, dehydration, overdiuresis, gastrointestinal bleeding, arrhythmia) should be considered and addressed before systematically decreasing guideline-directed medical therapy.
We would not initiate radical interventions in patients with asymptomatic or nonsevere hypotension (SBP 90 mm Hg or greater), as most patients with heart failure can tolerate guideline-directed medical therapy irrespective of low blood pressure measurements as long as volume status is adequately optimized. Transient asymptomatic blood pressure drops are common during guideline-directed medical therapy dosing but typically resolve with heart failure improvement. Determining association between low SBP and functionally limiting symptoms (eg, dizziness) is essential before initiating down-titration of guideline-directed medical therapy and can be readily assessed with orthostatic vitals.
Symptomatic hypotension
In severe (SBP less than 90 mm Hg) or symptomatic hypotension, any drug that lowers blood pressure and is otherwise not indicated in patients with heart failure (eg, calcium channel blockers) should be immediately stopped. Lastly, in case of refractory hypotension, diuretics may also be tapered in the absence of prominent congestion. Volume assessment may frequently be challenging, and it would be reasonable to consider right-heart catheterization for a more accurate assessment.
INITIATION OF GUIDELINE-DIRECTED MEDICAL THERAPY
Hospitalization of patients with acute decompensated heart failure provides an opportunity to initiate and continue guideline-directed medical therapy before discharge. Nonetheless, prolonging the hospital stay for optimization of guideline-directed medical therapy may not be cost-effective, and long-term benefits are only realized through outpatient adherence.24 Accordingly, we do not recommend the extended conventional approach to guideline-directed medical therapy optimization (ie, the guideline-directed medical therapy sequence followed in clinical trials), but rather advocate for rapid escalation of guideline-directed medical therapy owing to the following reasons.24
The addition of multiple agents has been shown to provide substantially more benefit, even at lower-than-target doses, compared with up-titrated single agents24,26
The beneficial effects of each class of guideline-directed medical therapy are independent of others24
Acute decompensated heart failure represents a high-risk period for patients with associated high morbidity and mortality, and guideline-directed medical therapy reduces adverse events as early as 30 days after readmission, thereby minimizing delay in benefits24
Prescription of guideline-directed medical therapy at the time of hospital discharge increases adherence in the outpatient setting.24 Early initiation of guideline-directed medical therapy in hypotensive patients with “warm and wet” hemodynamic profiles is generally feasible.24
However, patients who remain hypotensive despite optimization of volume status or those who develop disproportionately worse kidney function when attempting guideline-directed medical therapy titration may benefit from right heart catheterization-guided management.27,28 According to in-hospital initiation trials, guideline-directed medical therapy should be initiated once SBP is stable for 6 hours (ie, no increase in the intravenous diuretic dose for 6 hours, no intravenous vasodilators including nitrates within the prior 6 hours, and no intravenous inotropic drugs for 24 hours).24,29,30
Our approach
SGLT-2 inhibitors are very well tolerated in acute decompensated heart failure because of negligible hypotensive effects, but their natriuretic properties may require diuretic dose reduction.31 Likewise, mineralocorticoid receptor antagonists have minimal effects on blood pressure.32 In our experience, mineralocorticoid receptor antagonist dose reductions or alternate day dosing can be considered with potassium levels of at least 5.5 mEq/L. Beta- blockade and aldosterone antagonism—via ARN inhibitors, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers (ARBs)—have shown the greatest impact on morbidity and mortality in patients with acute decompensated heart failure and should be first-line in stabilized patients with heart failure.33 However, we would recommend introducing target-dose mineralocorticoid receptor antagonists and SLGT-2 inhibitors first in acute decompensated heart failure with symptomatic or clinically significant hypotension.
While in-hospital ARN inhibitors appear both effective and safe even with lower baseline SBP levels during acute decompensated heart failure,34 beta-blockers have less pronounced afterload-reducing properties.35 The presence of active ischemia, tachyarrhythmias, or specific cardiomyopathies (eg, cardiac amyloidosis) may also favor preferential use of beta-blockers.
Once the patient with hypotensive acute decompensated heart failure is already improving clinically, we would start a low dose of short-acting beta-blockers, such as metoprolol tartrate, which lacks the alpha-blocking properties of carvedilol, followed by gradual titration. If hypotension is suspected to be caused by a low cardiac output state, beta-blockers should be deferred to allow for compensatory tachycardia, and aldosterone antagonism via ACE inhibitors, ARBs, or ARN inhibitors may be carefully trialed first. Short-acting ACE inhibitors (eg, captopril) may be useful during the initial titration phase. To further minimize risk of recurrent hypotension during guideline-directed medical therapy titration, minimization of diuretics and appropriate spacing of guideline-directed medical therapy dosing are helpful.
Kidney function may deteriorate during early initiation of guideline-directed medical therapy compounded by intravenous diuretics.24 Nevertheless, renal function often stabilizes over time, and guideline-directed medical therapy has proven benefits even with an estimated glomerular filtration rate of 15 mL/minute/1.73 m2 in the context of chronic kidney disease. Initiation of ARN inhibitors or SGLT-2 inhibitors require higher estimated glomerular filtration rates.24
CONTINUATION OF THERAPY IN PATIENTS WITH HYPOTENSION AND ACUTE DECOMPENSATED HEART FAILURE
On the other hand, guideline-directed medical therapy down-titration should be considered once reversible causes have already been addressed. It is worth noting that abrupt withdrawal of beta-blockers, ACE inhibitors, ARBs, or ARN inhibitors may lead to clinical decline, and therefore should never be done in the absence of symptomatic hypotension or end-organ damage. As a rule of thumb, medications with less benefit for mortality rates (eg, hydralazine, isosorbide, or mineralocorticoid receptor antagonist) should be temporarily stopped first.
Beta-blockers should be temporarily stopped in the presence of symptomatic bradycardia, while aldosterone antagonists (mineralocorticoid receptor antagonists, ACE inhibitors, ARBs, or ARN inhibitors) may be stopped mainly in the setting of acute kidney injury or potassium of at least 5.5 mEq/L. Similarly, a history of arrhythmias should warn against beta-blocker interruption in favor of an ACE inhibitor, ARB, or ARN inhibitor taper. A similar approach to these common heart failure phenotypes has been proposed also for patients with ambulatory heart failure.35 Regardless of the clinical phenotype, arranging for early post-discharge follow-up for ongoing medication titration is mandatory for long-term success.
THE BOTTOM LINE
It is important to recognize that intolerance to guideline-directed medical therapy remains a poor prognostic indicator, and referral to the advanced heart failure teams would be warranted to explore candidacy for advanced therapies for patients.
Risk-stratifying patients with acute decompensated heart failure by focusing on avoidance, early recognition, and management of symptomatic and clinically significant hypotension results in the most promising outcomes for these patients.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest
- Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.