ABSTRACT
In teaching and in practice, little attention is given to a low anion gap. This oversight can result in a missed opportunity to diagnose acute or chronic disorders requiring treatment. In this article, we review the constituents of the anion gap, build a differential diagnosis for a low anion gap using case examples, and provide a stepwise approach to diagnostic testing to evaluate this abnormal finding.
Testing error is the most common reason for a low serum anion gap, so a patient should first undergo repeat serum electrolyte sampling to confirm the finding.
A decrease in negatively charged albumin lowers the anion gap. The effects of hypoalbuminemia on the anion gap can be accounted for using a correction formula. This correction is important because hypoalbuminemia may conceal an elevated anion gap metabolic acidosis.
An increase in an unmeasured cation, such as lithium, can lead to a low or negative anion gap. With early recognition, supratherapeutic lithium can be removed through hemodialysis.
An increase in a positively charged plasma protein lowers the anion gap; therefore, a low anion gap should prompt consideration of a monoclonal gammopathy.
In teaching and in practice, when we talk about the anion gap, we usually focus on recognizing and evaluating an elevated anion gap, which frequently requires immediate intervention. Little attention, however, is given when a patient presents with a low or negative anion gap. Such oversight can result in a missed opportunity to diagnose acute or chronic disorders requiring treatment.
In this article, we review the constituents of the serum anion gap, outline a differential diagnosis for a low anion gap using case examples, and provide a stepwise approach to diagnostic testing for clinicians to evaluate a patient that presents with a low anion gap. This is not an exhaustive review of acid-base physiology,1 but rather a practical starting point for generalists encountering this clinical problem.
WHAT MAKES UP THE ANION GAP?
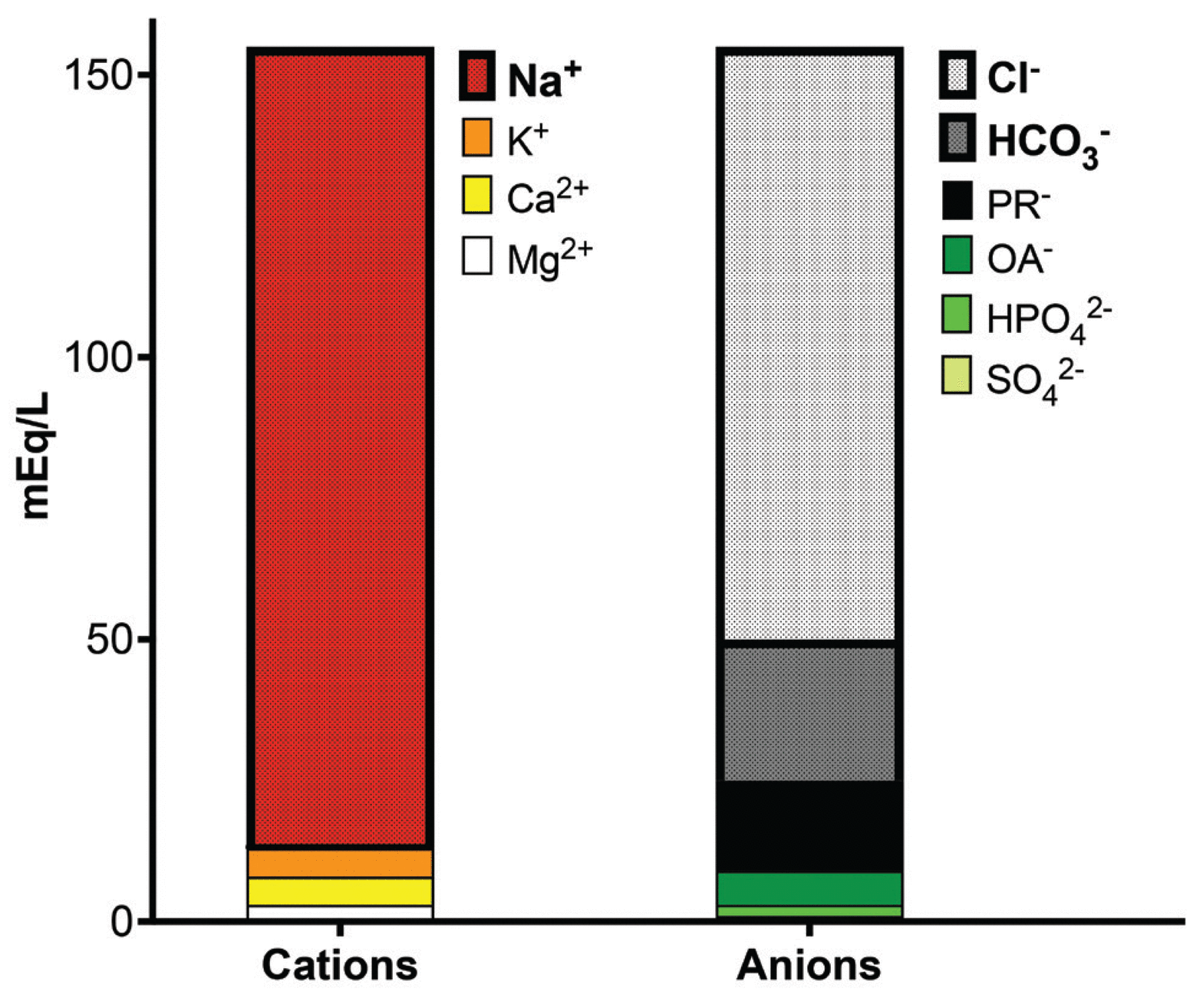
The serum contains an equal number of positively charged cations and negatively charged anions. A “Gamblegram,” named after physician James L. Gamble, offers a visual representation of each negatively and positively charged extracellular ion in the serum (Figure 1).
A “Gamblegram” showing the relative abundance of extracellular anions and cations. The serum cations are Na+ (sodium), K+ (potassium), Ca2+ (calcium), and Mg2+ (magnesium). The serum anions are 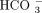 (bicarbonate), Cl−(chloride),
(bicarbonate), Cl−(chloride), 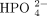 (hydrogen phosphate),
(hydrogen phosphate), 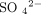 (sulfate), OA− (organic acids), and PR− (proteins). In this chart and in this article, for consistency, we use milliequiva-lents per liter (mEq/L) as the unit of measurement.
(sulfate), OA− (organic acids), and PR− (proteins). In this chart and in this article, for consistency, we use milliequiva-lents per liter (mEq/L) as the unit of measurement.
Note: Laboratories report anion gap in either mEq/L or millimoles per liter (mmol/L): 1 mEq/L is equal to 1 mmol/L multiplied by the valence charge of the ion. Since the anion gap calculation involves only variables with a valency of +1 or −1 (sodium, bicarbonate, chloride), the value in mEq/L will be identical to the value in mmol/L.
The anion gap is calculated by subtracting the sum of the serum chloride and bicarbonate concentrations from the serum sodium concentration (Table 1). The serum potassium contributes little to the total extracellular electrolyte pool and is often excluded from the calculation.2 The value for sodium is nearly always higher than the combined value of chloride and bicarbonate, leading to the typically positive anion gap value.
Three formulas for the anion gap
HOW LOW IS TOO LOW?
Until the 1980s, the reference range for the anion gap was between 8 and 16 mEq/L. When new serum electrolyte assays were introduced, the reference range was revised to between 3 and 9 mEq/L.3 A low anion gap is defined as less than or equal to 3 mEq/L.4
CAUSES OF A LOW ANION GAP: CASE SCENARIOS
Testing error
A healthy 30-year-old patient has a chemistry panel measured during a routine checkup. The anion gap is 2 mEq/L.
Measurement error in serum chemistry is the most common cause of a low anion gap.5,6 The anion gap is a derived number, dependent on 3 variables: the sodium, chloride, and bicarbonate concentrations. A preanalytical or analytical error in any of these can lead to a falsely low anion gap.7,8
Preanalytical errors arise during sample collection, transportation, and storage.9 Analytical errors arise from measurement processes or poor quality control in the laboratory. If previous tests do not demonstrate a low anion gap, the patient should undergo repeat serum electrolyte sampling to exclude testing process error.
Decrease in an unmeasured anion (albumin)
A 50-year-old with a recent diagnosis of gastric cancer is admitted for nutritional support. The patient reports minimal oral intake over 2 months and appears cachectic. Laboratory values on admission and on serial measurement show an albumin of 2 g/dL and an anion gap of 2 mEq/L.
Negatively charged plasma proteins make up most of the unaccounted anions in the anion gap calculation, and albumin is the most abundant of these proteins. Therefore, serum albumin comprises most of the normal anion gap:

Serum albumin concentrations may decrease due to malnutrition, impaired hepatic synthesis, acute or chronic inflammation, and urinary or gastrointestinal losses.10 When albumin falls, so does the anion gap, as chloride anions rise to compensate for the loss of albumin anions to maintain serum electrical neutrality.11
Multiple studies have demonstrated a correlation between hypoalbuminemia and the anion gap. One published correction formula for hypoalbuminemia adjusts a patient’s anion gap by adding 2.5 mEq/L to the calculated anion gap for each decrease of 1 g/dL in albumin from a normal baseline of 4 g/dL12:
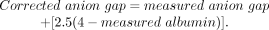
Our patient’s anion gap of 2 mEq/L corrects to a normal value of 7 mEq/L when adjusted for a serum albumin of 2 g/dL.
Hypoalbuminemia can conceal an emergency
A 50-year-old patient with a gastric ulcer presents with acute abdominal pain. Laboratory testing shows an albumin level of 2 g/dL and an anion gap of 13 mEq/L.
When we adjust for the patient’s albumin concentration of 2 g/dL and add 5 mEq/L to the calculated anion gap, the patient’s corrected anion gap rises to 18 mEq/L. This elevated level may prompt targeted testing for an anion gap metabolic acidosis, including lactate measurement, abdominal imaging, or surgical consultation. Omitting this adjustment for albumin could lead the clinician to miss a metabolic acidosis indicative of an intra-abdominal catastrophe, such as a perforated viscus.13
Increase in an unmeasured cation
A 50-year-old patient with bipolar affective disorder presents with obtundation after an overdose of home medications. Admission laboratory tests and repeat measurements show an anion gap of 0 mEq/L.
Increases in cations such as potassium, magnesium, and calcium can lower the anion gap. However, increases in these electrolytes are usually noticed on direct measurement before reaching the point of markedly affecting the anion gap calculation.
Other cations that are not routinely measured, such as lithium, can result in a low or negative anion gap.14 The excess positive lithium ions are balanced by a compensatory increase in negatively charged chloride ions. The sodium level remains the same, but the increased chloride causes a decrease in the calculated anion gap. In the right context, a low or negative anion gap should prompt serum lithium testing. With early recognition, supratherapeutic lithium can be removed through hemodialysis.15
Increase in a positively charged protein
A 70-year-old patient presents because of fatigue while riding a bicycle. Serial laboratory testing shows anemia, renal insufficiency, and an anion gap of 2 mEq/L.
A rise in positively charged plasma proteins can reduce the anion gap. Elevated positively charged proteins are counterbalanced by a compensatory increase in negatively charged ions, principally chloride. As chloride rises, the calculated anion gap falls.
The most common excess positively charged proteins are monoclonal immunoglobulins or light chains. Plasma cell dyscrasias such as multiple myeloma can produce positively charged immunoglobulin. The immunoglobulin G paraprotein has an isoelectric point higher than physiologic pH, resulting in a positive charge.16 A low anion gap should therefore prompt investigation for a monoclonal gammopathy.
Conditions that cause polyclonal increases in immunoglobulin levels can exert similar effects on the anion gap if a portion of these immunoglobulins are positively charged. Patients with higher levels of circulating immunoglobulins, including those with chronic kidney disease,17 cirrhosis,18 and human immunodeficiency virus,19 demonstrate an inverse correlation between their serum immunoglobulin levels and anion gap.
Chloride overestimation
A 70-year-old patient with osteoarthritis presents with nausea and tinnitus. The patient takes no medications other than over-the-counter aspirin for arthritis pain. Laboratory testing on admission and repeated during hospitalization shows a serum chloride concentration of 115 mmol/L and an anion gap of 0 mEq/L.
Chloride overestimation can occur when other halide ions such as bromide or iodide are erroneously read as chloride by ion-selective electrodes.20 While these agents are infrequently used in modern practice, they can still be found in sedative agents or in combination with nonsteroidal anti-inflammatory drugs. Increased salicylate levels, as in the patient above, may also mistakenly register as chloride when newer chloride ion-selective electrodes are used.21
Pseudohyperchloremia from these conditions results in an overestimated chloride level subtracted from an accurately recorded sodium value, leading to a decreased anion gap. In the right clinical context, a low anion gap should prompt investigation for bromide, iodide, or salicylate ingestion. The elevated anion gap acidosis typical of salicylate poisoning may be masked by this artifactually low gap.21
Sodium underestimation
A 70-year-old patient presents for replacement of a dislodged gastrostomy feeding tube, which led to no enteral intake for 3 days. Laboratory tests on admission and repeated during hospitalization show a serum sodium concentration of 170 mmol/L and an anion gap of 0 mEq/L.
Hypernatremia can occur through impaired access to free water or urinary loss of free water. In severe cases, the patient’s serum sodium concentration may exceed the upper limit of the laboratory assay (typically around 170 mmol/L), causing underestimation of the true sodium level. Underestimation of serum sodium can also occur in hyperviscosity states such as hyperproteinemia or hyperlipidemia due to difficulty with aspirating an adequate serum aliquot, which can be resolved by direct potentiometry.22 In cases of pseudohyponatremia, accurately recorded chloride and bicarbonate values are subtracted from an underestimated sodium value, resulting in a decreased anion gap.
AN APPROACH FOR CLINICIANS
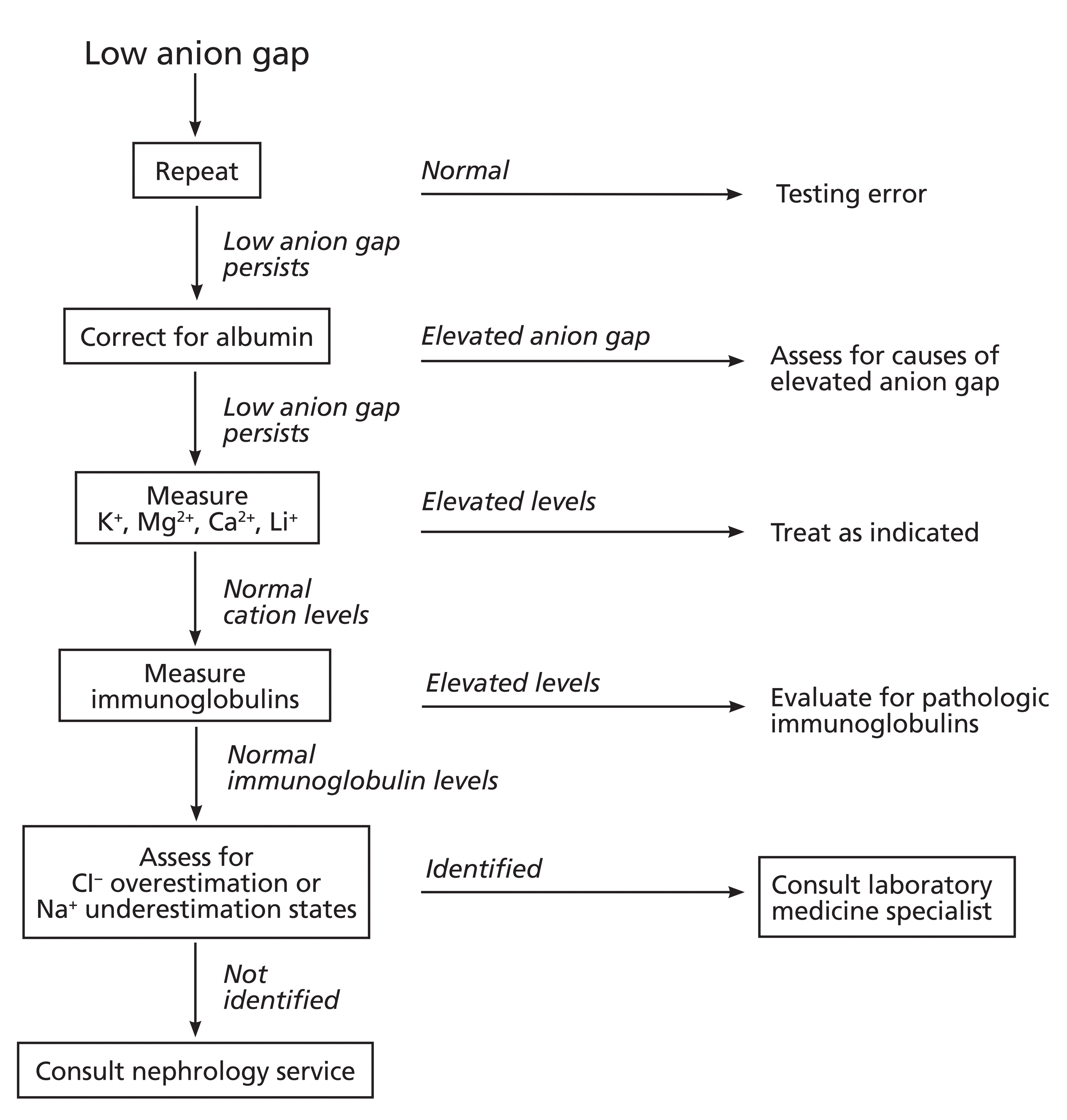
By understanding the components of the anion gap and how diseases and measurement techniques can affect the calculation, clinicians can direct initial diagnostic steps for a patient presenting with a low or negative anion gap (Figure 2). Nephrologists and laboratory medicine consultants are valuable collaborators for challenging or unexplained cases.
Stepwise approach to evaluating a low anion gap.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Acknowledgment
With appreciation for the interest in the topic generated by the late Dr. Richard Haber.
- Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.