Renal replacement therapies are the mainstay of treatment for uremic pericarditis and should be initiated as soon as possible. But when symptoms are refractory or fail to improve, pharmacologic therapies should be considered.
Uremic pericarditis, a condition with significant morbidity and mortality, was common at one time and initially reported in as many as 41% of patients with end-stage renal disease (ESRD) undergoing dialysis.1–3 With advancements in dialysis methods and earlier initiation of dialysis, the incidence has been reduced to approximately 5%, although this is still considerable given the number of people with ESRD.1,4
Uremic pericarditis is distinguished from dialysis-associated pericarditis based on the timing of clinical signs and symptoms of pericarditis in relation to renal replacement therapy. Uremic pericarditis is defined as the onset of clinical signs and symptoms of pericarditis before renal replacement therapy or within 8 weeks of initiation, and dialysis-associated pericarditis involves the onset of clinical manifestations after 8 weeks of renal replacement therapy.4 This is an arbitrary temporal designation and reflects the belief that dialysis-associated pericarditis is predominantly related to inadequate dialysis.5
PATHOPHYSIOLOGY OF UREMIC PERICARDITIS
The pathophysiology of uremic pericarditis is thought to involve metabolic alterations including hypoproteinemia, hyperuricemia, hypocalcemia, hyperparathyroidism, and accumulation of other toxic metabolites that exacerbate endothelial permeability.5,6 Dialysis-associated pericarditis is further highlighted in patients with inadequate dialysis secondary to lack of adherence or low-flow rates related to access issues or higher catabolic states.4 Circulating immune complexes have been implicated as pro-inflammatory toxins responsible for serositis, which is not specific to the pericardium.7
In addition to the inflamed pericardium, uremia places patients at a higher risk of bleeding and coagulopathy as a result of platelet dysfunction, an altered coagulation cascade, and activation of the fibrinolytic system.6 However, studies have not found a relationship between the degree of azotemia (or biochemical abnormalities) and the development of uremic pericarditis or dialysis-associated pericarditis.4 There are few adequate animal models for pericarditis, further challenging our understanding of the development of a pathophysiologic mechanism. A recently developed mouse model using inflammasome activation highlights the potential for biologic agents.8
SIGNS AND SYMPTOMS
Clinical features of uremic pericarditis include chest pain that typically occurs in the anterior chest, particularly in the recumbent position, that worsens with inspiration and can be associated with a pericardial rub, which is common in patients with uremic pericarditis and present in up to 83% of episodes.1,3,4,9 In severe cases, cardiac tamponade may be present in up to 16% of patients with dialysis-associated pericarditis.10 Therefore, the initial evaluation should involve excluding tamponade along with assessment for acute coronary and aortic syndromes, as patients on dialysis are at higher risk for major cardiovascular events.3
The diagnosis may be corroborated by findings on electrocardiography such as widespread concave ST elevation with PR depression, reciprocal ST depression, and PR elevation in lead aVR.11 In the case of pericardial effusion, low-voltage QRS complexes and classic electrical alternans may be found. Sinus tachycardia is a common but nonspecific finding, reflecting pain or a preload-dependent state. Overall, analysis has demonstrated specificity but minimal sensitivity of these findings, limiting their clinical utility.11 Echocardiography characterizes pericardial effusion but has limited utility for detailed pericardial assessment. Cardiac computed tomography and cardiac magnetic resonance imaging have become increasingly adapted to identify morphologic features of pericardial inflammation.
In the case of a pericardial effusion requiring drainage, pericardial fluid analysis may provide additional diagnostic information.12 Uremic effusions are generally transudative, while exudative effusions could suggest either hemorrhagic conversion or an underlying systemic inflammatory disorder that contributed to renal injury (such as glomerulonephritis related to vasculitis or systemic lupus erythematosus).
RENAL REPLACEMENT THERAPY
In treating uremic pericarditis, the removal of uremic toxins entails either initiation of dialysis in patients with chronic kidney disease or intensification of dialysis in those with ESRD.1 There is no known difference in response to dialysis in patients with uremic pericarditis than in those with dialysis-associated pericarditis, although the 2 entities differ in that 1 requires initiation of dialysis while the other depends on the technical features of the dialysis method.
For patients without an adequate response to the initiation of dialysis, intensifying the frequency (to 5 to 7 days a week) or the duration of chronic dialysis is recommended.9 In patients with dialysis-associated pericarditis, adequate dialysis dosing is imperative, and this includes ensuring adherence and adequate access flow, as well as addressing access issues. Resolution of clinical pericarditis has been reported to occur in 87% of patients within 2 weeks of starting chronic dialysis.9
There may be differences in removal of relevant toxins between hemodialysis and peritoneal dialysis. A small case series demonstrated improvement in patients with pericarditis and hemorrhagic effusions refractory to appropriate hemodialysis once peritoneal dialysis was initiated.13
Complications
While the rate of hemorrhagic pericardial effusion is low, systemic anticoagulation should be avoided when possible owing to the risk of hemorrhagic conversion, especially in the context of possible uremic platelet dysfunction, which can be difficult to quantify with routine laboratory assessment.1,12 In the context of myocardial infarction treated with anticoagulation, older series have demonstrated a higher rate of hemopericardium, though incidence and guidance for modern anticoagulation methods are less clear.14,15
In patients presenting with severe complications of uremia (eg, encephalopathy, severe refractory acidosis, symptomatic pericardial effusion) and high degrees of azotemia, dialysis needs to be initiated slowly, with low flow rates to avoid disequilibrium syndrome. Meanwhile, in patients with larger pericardial effusions, judicious ultrafiltration must be done with close hemodynamic monitoring to ensure adequate cardiac filling.
GUIDING THERAPY
It is important to note the progression of techniques and evaluation of dialysis over time and various reasons for considering transition of modality. While there are no standard clinical or laboratory criteria to determine the success of dialysis, intensive dialysis should be continued until resolution of symptoms and resolution of pericardial friction rub. Multimodality imaging is increasingly used to assess pericardial disease, and imaging-guided therapies are used in cases of clinical suspicion for pericarditis without obvious findings of an associated effusion on echocardiography.12,16 These methods provide quantitative and qualitative data on pericardial disease and can elucidate underlying causes.
Late gadolinium enhancement and T2 short tau inversion recovery sequencing in magnetic resonance imaging are of particular interest when assessing pericardial and myocardial inflammation. Emerging data in recurrent pericarditis support modifying therapies in response to findings on cardiac magnetic resonance imaging, particularly in patients taking multiple anti-inflammatory therapies that can falsely decrease inflammatory markers.16 Serial follow-up imaging studies can be compared along with serologic measures of inflammation (C-reactive protein and erythrocyte sedimentation rate) to assess the adequacy of therapy, together with careful clinical assessment. This cardiac magnetic resonance imaging-guided response to therapy allows for the tailoring of treatment strategies in response to pericardial inflammation and edema resolution.16 Additionally, factors such as low systolic blood pressure, leukocytosis, high-grade fever, and large pericardial effusions have been reported as predictors of dialysis failure.17
PERICARDIAL INTERVENTIONS
Infrequently, pericarditis remains refractory to intensive dialysis treatment. If patients develop tamponade physiology or pericardial effusions do not improve within 2 weeks of intensive dialysis, pericardial drainage is indicated.1,12 Patients with a large pericardial effusion—especially if associated with tamponade physiology—are not ideal candidates for urgent dialysis because of potential hemodynamic effects of ultrafiltration. In these situations, a pericardial window is a useful temporizing strategy before ultrafiltration and toxin removal can be achieved. Pericardiocentesis may be safely performed under echocardiographic guidance, with a 1.2% rate of major complications.18 Nonetheless, the introduction of the often unnecessary risk and insufficient durability of needle drainage has led to the procedure being largely reserved for acutely unstable patients as a bridge to surgical drainage.
A pericardial window procedure is usually preferred over the high-risk formal pericardiectomy.12 While a pericardial window offers the advantage of obtaining pericardial biopsy to rule out other causes of pericarditis, it does not eliminate pericardial inflammation until the uremic state is resolved with simultaneous dialysis. In patients with constrictive pericarditis or large recurrent pericardial effusions despite pericardial drainage, pericardiectomy serves as definitive therapy.
PHARMACOLOGIC THERAPY IN PATIENTS WITH RESIDUAL KIDNEY FUNCTION
When symptoms are refractory or fail to improve with maximally tolerated dialysis, pharmacologic options for uremic pericarditis are limited by their nephrotoxicity (in patients with residual renal function or possible renal recovery), the need for dosing adjustments, and bleeding risk.12 Unlike other forms of pericarditis, first-line anti-inflammatory therapies such as non steroidal anti-inflammatory drugs are generally avoided in patients who are not dialysis-dependent, especially in high-dose regimens. However, they may be used at the lowest effective dose for the shortest possible duration. The European Society of Cardiology guidelines include a class III recommendation against the use of colchicine in patients with advanced kidney disease,12 and a creatinine clearance cutoff of 30 mL/minute is usually adopted.19 Corticosteroids have been used with varying benefit, with low doses mainly considered in patients unable to use non steroidal anti-inflammatory drugs.
CONSIDERATIONS IN END-STAGE RENAL DISEASE WITHOUT RESIDUAL KIDNEY FUNCTION
In patients with declared ESRD in whom worsening renal function is not necessarily a concern, there are still multiple issues that can be concerning, such as drugs that may be variably cleared through dialysis, significantly reducing efficacy. In patients with uremic platelet dysfunction, bleeding is an important concern, particularly when pericardial effusions are present, as is the risk for hemorrhagic conversion. Further, patients with advanced chronic kidney disease often have multiple comorbidities, experience worsening of concomitant coronary artery disease or heart failure, and have difficulty with volume and blood pressure management due to corticosteroids. These examples demonstrate how traditional treatment strategies involve risk and emphasize the need for nonpharmacologic and alternative therapies in this vulnerable population.
BIOLOGIC AGENTS
Newer therapies for the management of recurrent pericarditis including anakinra and rilonacept have not been robustly explored for use in patients with uremic pericarditis.
Anakinra is not dialyzable, but there is a recommendation for every-other-day dosing in patients with a creatinine clearance rate less than 30 mL/minute.20 This adjustment is based on pharmacokinetic studies and aims to reduce the development of drug-neutralizing antibodies, infection from immunosuppression, and gastrointestinal side effects including hepatotoxicity.20
Rilonacept does not appear to need dose adjustment in patients with impaired kidney function.21 It is worth noting that residual cardiovascular risk in patients with impaired kidney function appears to be driven significantly by inflammation, as has been quantified with measurements of high-sensitivity C-reactive protein and interleukin-6.21
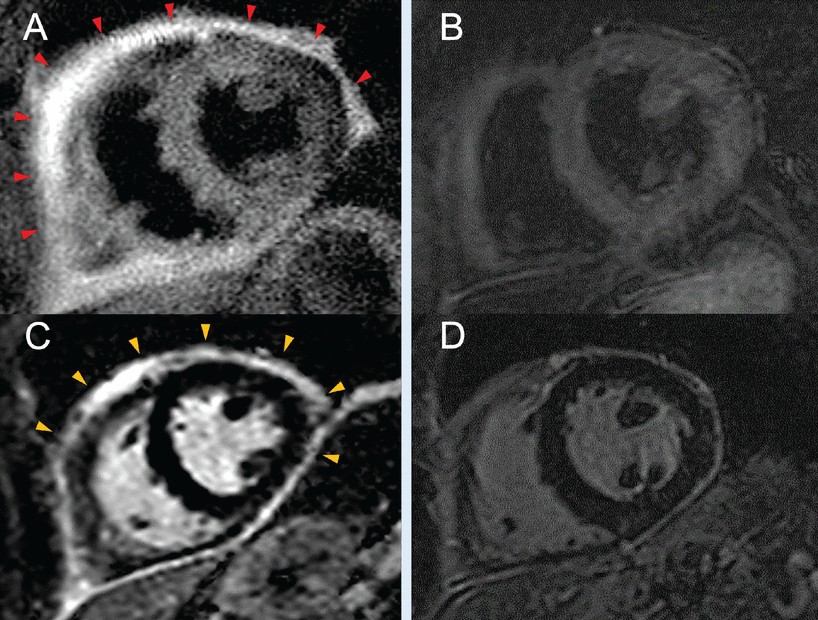
With this in mind, the role for targeted immunomodulatory therapies in the treatment of uremic pericarditis needs further study. However, these agents have already shown promising results in the management of recurrent pericarditis, with substantial decreases in pericardial inflammation and resolution of edema on cardiac magnetic resonance imaging (Figure 1).
(A) Cardiac magnetic resonance imaging with T2 short tau inversion recovery sequencing shows increased signal intensity (red arrowheads) before initiation of anakinra and (B) while on anakinra, with no evidence of edema. (C) Cardiac magnetic resonance imaging with contrast shows severe late gadolinium enhancement (yellow arrowheads) before starting anakinra, and (D) significant improvement in late enhancement after anakinra.
THE BOTTOM LINE
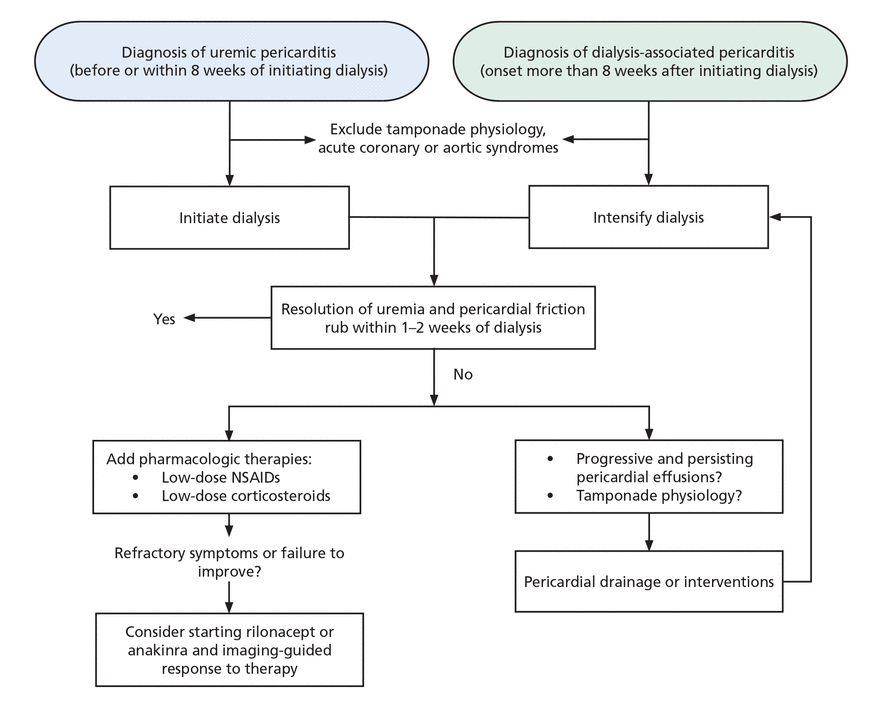
Management of uremic pericarditis requires a thoughtful, multidisciplinary approach that involves the patient and a team of internal medicine, nephrology, and cardiology clinicians. Renal replacement therapies are the mainstay of treatment and should be initiated as soon as possible. Pharmacologic therapy should be deferred initially because of the risk of side effects and the unclear evidence regarding efficacy prior to adequate dialysis. When symptoms are refractory or fail to improve, pharmacologic therapies should be considered (Figure 2).
Proposed algorithm for management of uremic pericarditis.
NSAIDs = nonsteroidal anti-inflammatory drugs
DISCLOSURES
Dr. Klein has disclosed serving as advisor or review panel participant for Cardiol Therapeutics, Kiniksa Pharmaceuticals, and Pfizer; consulting for Kiniksa Pharmaceuticals and Pfizer; and intellectual property rights with Elsevier and Wolters-Kluwer. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.