ABSTRACT
In its current global outbreak, mpox has exhibited several novel clinical presentations that clinicians should be aware of so they can recognize it if they see it. Although the case rate has decreased, mpox could linger at a low rate or resurface in other populations and thus should remain in the differential diagnosis in patients presenting with potential infections after intimate encounters.
In its worldwide outbreak in 2022, mpox was remarkably different from its historic profile, a viral zoonotic disease that inefficiently spread from person to person.
Mpox is currently primarily affecting men who have sex with men and is mainly transmitted through direct contact with an infectious lesion.
Clinicians should keep mpox in the differential diagnosis for single, multiple, or diffuse genital, anal, or skin lesions, as well as pharyngitis and proctitis.
Patients with suspected mpox should also be tested for sexually transmitted infections including human immunodeficiency virus (HIV), and should be offered HIV postexposure or preexposure prophylaxis and mpox vaccine if appropriate.
Mpox, formerly known as monkeypox, is a viral zoonotic disease caused by the mpox virus. This review describes the epidemiology of the 2022 mpox outbreak, the clinical presentation and differential diagnosis of mpox, and its management and prevention.
RELATED TO SMALLPOX
The mpox virus is a double-stranded DNA virus in the genus Orthopoxvirus, family Poxviridae. This genus encompasses many poxviruses, including some that infect humans exclusively, some that infect various animal species exclusively, and some that are zoonotic. Other medically important orthopoxviruses include variola (the causative agent of smallpox, which was eradicated from nature in 1980), vaccinia (source of the modern smallpox vaccine), and cowpox (used by Jenner in 1796 to induce immunity to smallpox through inoculation).
There are two clades (subtypes) of mpox virus that have historically been described in different regions of Africa since the 1970s. Clade I virus has been responsible for zoonotic mpox disease in the Congo basin (Central Africa) and is thought to be more virulent, with mortality rates of approximately 10%. In West Africa, where clade II virus is the causative agent, the mortality rate has historically been low, less than 1%.1
SHIFTING EPIDEMIOLOGY
Animals to people
The epidemiology of mpox has shifted. From the 1970s, when it was first recognized in humans, until the early 2000s, mpox was an endemic zoonotic disease occurring sporadically in the rain forests of West and Central Africa among people who had direct contact with forest animals such as monkeys, rodents, and squirrels. Documented person-to-person spread was infrequent and usually occurred among close family members.2
Then, from 2005 to 2007, the incidence of mpox increased 20-fold in the Democratic Republic of the Congo, when 760 laboratory-confirmed cases were identified.3 It was proposed that the increase was due to waning immunity levels in the population, who were no longer being vaccinated against smallpox. Smallpox vaccination, which provides cross-protective immunity against mpox, was discontinued in the Democratic Republic of the Congo in 1980 after a successful vaccination campaign in which 24.3 million people were vaccinated from 1968 through 1971, resulting in smallpox eradication in the region in 1971.4 An active disease-surveillance program during this time found that the risk of mpox was 5.21 times lower in persons vaccinated against smallpox than in unvaccinated persons.3
Sporadic travel-associated cases of mpox were also reported outside of endemic countries during this time. The largest outbreak outside of Africa was in 2003, with 71 cases in the midwestern United States associated with importation of mpox-infected rodents from Ghana and spread of the infection to pet prairie dogs exposed in a distribution center.5
Person to person
A harbinger was seen in Nigeria in 2017, when mpox re-emerged 39 years after the last reported case of it there. Unlike earlier outbreaks, this occurred in a primarily young adult male population living in urban and periurban environments, and there was suspected frequent human-to-human transmission. During a 1-year period, Nigerian scientists identified 118 laboratory-confirmed cases, and while the specific manner of transmission was not addressed, they noted that of 65 patients with information available, 44 (68%) had genital lesions.6 While sexual transmission was not suggested directly in the original report, the fact that homosexuality is a felony offense in Nigeria may have prevented an open discussion of this mechanism.
The 2022 worldwide outbreak
In May 2022, mpox was detected in multiple countries in Europe where it is not endemic. The specific etiology of the outbreak has not been fully elucidated. Many of the early cases were linked to an international gay pride event and occurred primarily among men who reported having sex with multiple male partners.7 The virus was genetically similar to the clade II virus that caused the 2017 outbreak in Nigeria.8
The first case of mpox in the United States was recognized on May 17, 2022, in Massachusetts, and more cases were ultimately found in all 50 states over the subsequent months. The peak of the US outbreak was in early August, when the US Centers for Disease Control and Prevention (CDC) reported a 7-day average of 457 cases per day. As of June 23, 2023, there were 30,505 domestic cases and 88,026 worldwide.9
In the United States, the mpox outbreak has been highly concentrated in certain populations. By far, most cases (95.8%) have been in cisgender men, most of whom identify as gay, bisexual, or other men who have sex with men. Racial and ethnic minorities have been disproportionately affected including Black communities (31.1% of cases) and Latin-American communities (29.9% of cases).9 Geographically, most cases have occurred in US states with large urban areas, particularly those with substantial lesbian, gay, bisexual, transgender, and queer or questioning populations.
Usually sexually transmitted
People are exposed to mpox virus primarily through direct physical—often intimate—contact with infectious lesions. Less commonly, mpox is transmitted through fomites, usually among close household contacts.10 Animal models demonstrate that mpox, like smallpox, can also be transmitted through respiratory droplets,11 but the contribution of this route of transmission to the current outbreak is thought to be negligible.
During the current outbreak, direct physical contact with infectious material from skin lesions or mucous membranes during sexual activity is considered the main risk factor for acquisition. While viral DNA has been detected in semen, saliva, urine, and feces, it is unclear whether contact with these fluids transmits infection,12 but there is mounting epidemiologic evidence that people with presymptomatic and possibly asymptomatic mpox are playing a role in spreading the disease, including a study that suggests that transmission can occur without a visible rash.13
THE CLINICAL PRESENTATION HAS CHANGED
The clinical presentation of mpox during the current outbreak has differed from the classic presentation described in endemic countries over the past several decades. Classically, mpox has been a systemic illness characterized by fevers, chills, and myalgias accompanied by a characteristic diffuse, centrifugal rash consisting of well-circumscribed, deep-seated pseudopustules with central umbilication that were all in the same stage of development.
During the current outbreak, the clinical manifestations have been more protean. Key distinguishing features of the current outbreak are a wide range of severity of disease and, frequently, lesions at the site of inoculation.14
Recognizing mpox in immunocompetent patients
Patients with mpox may experience a range of symptoms, from asymptomatic isolated skin lesions without systemic illness to severe disseminated disease. In immunocompetent patients, infection tends to be less severe.
The incubation period can range from 4 to 21 days, with an average of 5.6 days from exposure to symptom onset and 7.5 days from exposure to rash onset.9 Prodromal symptoms are nonspecific and can include fever, lymphadenopathy, malaise, chills, pruritus, headache, myalgias, nausea, vomiting, or abdominal pain. Most patients experience at least 1 systemic symptom during their disease, but a minority have none.
The rash usually appears 1 to 2 days after prodromal symptoms begin. Of note: the appearance and distribution of the rash varies widely in the current outbreak. Patients may have a single lesion or multiple lesions at a single site (usually the site of inoculation), or disseminated lesions involving the extremities, trunk, or face. The lesion typically starts as a 2- to 5-mm red macule, progressing to a papule, then a vesicle, then a pseudopustule (filled with cellular debris with high amounts of virus). Finally, the lesion crusts over and the crust dries and falls off. The period from macule to reepithelization can be up to 14 days in immunocompetent hosts. New lesions may appear during the course of the illness, and thus can exist in different stages of development (Figure 1).15
Mpox lesions in various stages of development: (A) early vesicle, (B) small pustule, (C) umbilicated pustule, (D) ulcerated lesion, (E) crusted mature lesions under the lower lip, and (F) partially removed scab.
Adapted from reference 15.
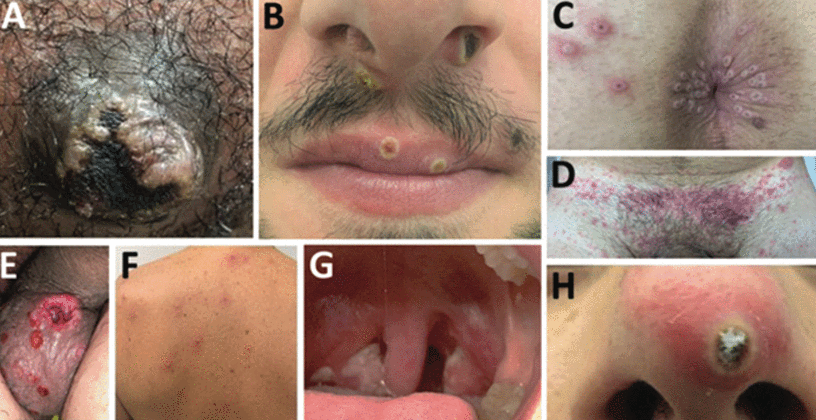
Given that the main route of transmission during this outbreak is through sexual contact, inoculation frequently occurs in the genital area, anus, rectum, or oropharynx (Figure 2).16
Sites of mpox lesions in an observational cohort study in southern France. (A) Primary inoculation site showing an irregular pustule with necrotic crust of the right nipple. (B) Pustular lesions with a crusted center on the mucosa of the upper lip, close to the left oral commissure and left nasal orifice. (C) Pustules circumferentially distributed on the anal margin and perianal skin of varying sizes and stages of evolution, some with central necrotic crusts. (D) Perineally extended purpuric lesions. (E) Scrotal lesions of varying sizes and stages of evolution, with edema surrounding the larger ulcero-hemorrhagic ulcers. (F) Scattered papules, pustules, and umbilicated pustules surrounded by an erythematous halo on the back. (G) Reddened and swollen right palatine tonsil with a fibrin-covered ulcer. (H) Pustular lesion on the nose with a necrotic central crust, whitish deposit, and erythematous halo.
Adapted from reference 16.
Genital lesions. When genital inoculation occurs, patients may develop single, few, or many lesions on the penis, scrotum, or pubis. The lesions are usually painful, but some patients report only mild itching or no symptoms. Most lesions heal without complication, but cases of severe edema leading to paraphimosis have been reported. Urethral involvement can lead to urethral strictures requiring urologic intervention. Confluent lesions can lead to ulcers or necrotic crusts.
Anal or rectal lesions. When inoculation occurs in the anus or rectum, patients may have external lesions on the buttocks, anal margin, or perianal skin that can cause significant pain with sitting or defecation. Isolated rectal mucosal disease without external rash has frequently been reported in men who have sex with men who participate in receptive anal intercourse. This manifests as proctitis, with symptoms that can include pain, tenesmus, and bloody or purulent discharge. Proctoscopy is usually not performed because it would be too painful, but friable tissue with pox lesions on the rectal mucosa has been described.17
Oropharyngeal lesions. If oropharyngeal inoculation occurs, patients may have visible external lesions on the lips, vermillion border, or perioral area. However, external visible lesions are not always present. Lesions in the posterior oropharynx or tonsils may be the only manifestation in patients who have oral exposure. This can lead to ulcerative pharyngitis or tonsillitis, or in rare cases mass lesions that can threaten to block the airway.
A link between mpox and HIV
Severe manifestations and poor outcomes have been reported in people living with human immunodeficiency virus (HIV), particularly those with advanced HIV infection and acquired immunodeficiency syndrome (AIDS). A November 11, 2022, report cited an HIV prevalence of 57% in adults diagnosed with mpox,18 compared with 0.36% in the general adult population.19 It is not yet known whether HIV infection affects an individual’s risk for acquiring mpox if the HIV infection is under control. It is plausible that there could be a biological mechanism for increased susceptibility to mpox in HIV-positive individuals, or that mpox and HIV both circulate in similar sexual-risk networks, thus increasing the overlap between the 2 conditions.
Severe mpox has often been reported in patients with low CD4 counts. A report from November 4, 2022, summarized findings from CDC clinical consultations for 57 patients hospitalized with severe mpox disease. Overall, 47 (82%) of the patients were living with HIV, but only 4 were receiving antiretroviral therapy, and 31 (72%) of 43 had a known CD4 count less than 50 cells per mm3.20 Lesions in such immunocompromised hosts are often enlarging and nonhealing.
As of March 7, 2023, the CDC received reports of 52 deaths in persons with confirmed or probable mpox, including 38 deaths that were classified as mpox-associated, 3 that were classified as non–mpox-associated, and 11 that remained under investigation. Among the 38 mpox-associated deaths, information was available for 33 patients, and 31 (94%) of them were immunocompromised due to uncontrolled HIV.21
Immunocompromising conditions other than advanced HIV infection may also predispose to severe mpox. The November 4, 2022, report20 noted severe disease in 2 patients undergoing chemotherapy for hematologic malignancy, 3 solid-organ transplant recipients, and 3 patients who were pregnant. Further investigations are needed to delineate the risk of severe disease in these populations.
Complications of severe mpox
Severe mpox can manifest as disseminated dermatologic disease with or without mucosal or organ involvement. In the 57 severe cases reported to the CDC,20 39 (68%) of the patients had mucosal lesions (oral, urethral, rectal, or vaginal), 12 (21%) had pulmonary disease, 12 (21%) had ocular disease, 5 (9%) had muscle or bone involvement, and 4 (7%) had neurologic disease. About one-third of patients required intensive care.
Complications of severe dermatologic disease can include bacterial superinfections, viral superinfections (most commonly with herpes simplex virus), and the need for surgical debridement of necrotic tissue. Viremia in mpox disease occurs during initial spread of systemic infection. With pulmonary involvement, mpox has a range of manifestations including pulmonary nodules, severe pneumonia, or empyema. Ocular involvement is also protean and can present as conjunctivitis, blepharitis, periocular cellulitis, keratitis, or subconjunctival nodules, and can result in loss of vision.
DIFFERENTIAL DIAGNOSIS
The clinical presentation of mpox overlaps with those of other viral infections and sexually transmitted bacterial infections. The flulike prodrome is nonspecific, so before skin or mucosal lesions appear, the clinician should keep mpox in the differential diagnosis in the right epidemiologic context by obtaining a relevant sexual and exposure history.
Molluscum contagiosum
The classic deep-seated umbilicated pseudopustule of mpox is similar in appearance to those caused by molluscum contagiosum virus, another member of the poxvirus family but in a different genus than the orthopoxviruses.
Molluscum contagiosum can involve the trunk, extremities, groin, and genitals, as with mpox. It can occur in healthy children, adolescents, and adults. In adults and sexually active adolescents, it can be transmitted by intimate contact, as with mpox. However, molluscum contagiosum lacks the prodromal symptoms and takes on a more chronic time course, with most infections self-resolving in 6 to 12 months. However, in immunosuppressed patients (particularly in advanced HIV infection), molluscum contagiosum can appear more rapidly and diffusely and persist, increasing the clinical overlap between molluscum contagiosum and mpox disease.
Herpesviruses
When mpox is in the vesicular stage of development it can be difficult to differentiate from infection with herpesviruses such as herpes simplex virus and varicella zoster virus.
To evaluate for herpes simplex virus, the clinician should ask about previous oral or genital herpes attacks, since patients with oral or genital herpes often experience multiple subsequent outbreaks. In patients with no history of oral or genital herpes, primary herpes simplex virus infection can present with a prodrome and rash at the site of inoculation associated with tender lymphadenopathy, similar to mpox. The time course and evolution of the rash may help differentiate the 2 diseases: herpes simplex virus lesions progress from vesicles to erosions and ulcerations, while mpox lesions progress to firm pseudopustules.
Infection with varicella zoster virus, which causes chickenpox and shingles, can also mimic mpox. Shingles classically manifests as systemic symptoms associated with a dermatomal rash of erythematous, grouped vesicles with acute neuritis. In immunocompromised individuals, disseminated varicella virus infection may be considered if they have a diffuse rash.
Any rash that cannot be clinically identified with certainty should be sampled for polymerase chain reaction (PCR) testing for orthopoxvirus, herpes simplex virus, and varicella zoster virus.
Syphilis
Mpox lesions can mimic the chancre lesion of primary syphilis, which is classically described as a painless papule at the site of inoculation that progresses to a 1- to 2-cm ulcer with a raised, indurated margin. Importantly, a chancre can appear at any site where inoculation occurs, including the perioral area and oropharynx. Disseminated mpox can mimic some manifestations of secondary syphilis including pustular syphilis. In immunocompromised patients, disseminated mpox can resemble malignant syphilis (lues maligna), a severe ulcerative form of secondary syphilis.
Mucosal manifestations
Isolated oropharyngeal mpox may be mistaken for bacterial tonsillitis or primary oral herpes, while mpox proctitis may be clinically indistinguishable from chlamydial proctitis (including lymphogranuloma venereum), gonococcal proctitis, or syphilitic proctitis.
Chancroid, others
A less common cause of genital ulcers is Haemophilus ducreyi, the causative agent of chancroid. The classic presentation of chancroid is a deep, undermined, purulent ulcer associated with painful inguinal lymphadenitis. Since 2011, fewer than 20 cases per year have been reported in the United States.
Other dermatologic conditions that manifest with pustules should be considered in the right clinical context. These include infectious causes such as disseminated gonococcemia and noninfectious causes such as eosinophilic folliculitis (particularly in those with advanced HIV), pustular psoriasis, and acute febrile neutrophilic dermatosis (Sweet syndrome).
TESTING FOR MPOX
Diagnostic testing should be performed in all cases of suspected mpox. This can be done through consultation with public health authorities or by sending swabs to commercial laboratories. PCR testing for orthopoxvirus DNA should be performed on lesion samples.
Lesions should be vigorously swabbed to collect skin cells. Unlike lesions in herpes simplex virus infection that are easily “unroofed” during swabbing, mpox lesions will not unroof, and one should not attempt to unroof them with sharp implements, since accidental infections have occurred after needle stick.22 If there are multiple lesions, samples should be taken from at least 2 lesions. If no skin lesions are present, samples can be taken from sites of symptoms like the rectum or oropharynx. Samples should be clearly labeled with the site of collection in the case of multiple specimens.
The role of skin biopsy is limited, given the ease of PCR testing, but could be considered if PCR testing is unavailable or inconclusive.
Cotesting for sexually transmitted infections
Patients with mpox are frequently co-infected with other sexually transmitted infections. A CDC report in the early months of the 2022 outbreak noted that 25% of patients with mpox also had chlamydia, 28% had gonorrhea, and 8% had syphilis.23 A review of mpox cases at our institution in Philadelphia showed a 52% seropositivity rate for current or prior syphilis and 21% co-infection with gonorrhea or chlamydia for those who underwent testing, and the rectal gonorrhea positivity rate was 31% (unpublished data).
Therefore, the evaluation for mpox should include testing for sexually transmitted infections including HIV and syphilis, and triple screening (urine, rectal, oropharyngeal sampling) for gonorrhea and chlamydia. We recommend the tests listed in Table 1 for all potential mpox patients. Gonorrhea and chlamydia testing should be based on anatomy rather than gender identity: screening recommendations for cisgender females should be extended to all transgender males and gender-diverse people with a cervix, and recommendations for cisgender males should be extended to all transgender females and gender-diverse people with male anatomy.
Our recommended screening for sexually transmitted infections in patients with mpox
MANAGEMENT
Supportive care for mild disease
Management of mild disease in immunocompetent patients is primarily supportive because many patients with mpox recover without medical intervention. Pain control is the main concern.
Over-the-counter medications such as acetaminophen or nonsteroidal anti-inflammatory drugs are recommended as first-line therapy. Topical steroids or anesthetics such as lidocaine can be considered for local pain relief, but should be used with caution on broken skin or draining wounds. Patients should use gloves when applying topical agents to avoid autoinoculation. Other adjunctive therapies can include oral antihistamines to control pruritus, or topical agents such as calamine lotion or petroleum jelly.
Prescription pain medications such as gabapentin or opioids can be considered for pain not controlled with the above interventions. However, the risk of unintended consequences of long-term use of opioids should be carefully considered.
For proctitis, stool softeners to reduce pain with bowel movements should be considered. Topical lidocaine and warm sitz baths with baking soda or Epsom salts may provide additional symptomatic relief, but patients should drain the bath and disinfect the tub after use. In severe cases, patients may require hospitalization for pain management.
For pharyngitis, patients can try rinsing the mouth with saltwater every 6 hours. Prescription analgesic mouthwash (sometimes called “magic mouthwash”) can also be used.24
Antiviral therapy for severe disease, or high risk of severe disease
Tecovirimat is an antiviral drug that inhibits the orthopoxvirus protein VP37, preventing viral exit from the host cell. Tecovirimat therapy should be considered for patients with severe disease or at high risk of it (Table 2). These recommendations may change as further research becomes available.
Indications for tecovirimat treatment in individuals with mpox
Studies are ongoing to determine the optimal duration of treatment. The current recommendation is to treat immunocompetent patients for 14 days, starting as soon as the infection is confirmed or if clinical suspicion is high. Dosing and counseling information for tecovirimat can be found in Table 3.
Dosing and patient counseling for tecovirimat
Because tecovirimat was originally developed as a treatment for smallpox to address bioterrorism concerns, US Food and Drug Administration approval was not sought for the treatment of mpox disease. Oral tecovirimat is currently available by a CDC expanded-access program through local health departments for those who cannot enter a clinical trial. To access tecovirimat through this program, clinicians or facilities need to register with the CDC.25 However, we recommend referring the patient to a clinical trial if possible, since additional data are needed on efficacy and other measures. Multicenter clinical trials to evaluate efficacy are in phase 3, including the National Institute of Allergy and Infectious Diseases-supported Study of Tecovirimat for Human Monkeypox Virus (STOMP).26
Advanced therapies
Patients with severe mpox disease should be managed in consultation with an infectious disease expert or the CDC mpox consultation team (CDC Emergency Operations Center: 770-488-7100).
Considerations for treating severe disease or risk for progression to severe disease include optimizing immune function by limiting immunosuppressive agents, initiating antiretroviral therapy for those with uncontrolled HIV, extending or repeating the tecovirimat course, or adding other antiviral medications such as cidofovir or brincidofovir, and vaccinia immune globulin intravenous. Trifluridine eye drops should be used for ocular involvement.
Guidance for treatment of severe mpox is being updated as more information becomes available, and current recommendations can be found on the CDC website.27
INFECTION CONTROL IN HEALTHCARE SETTINGS
In both inpatient and outpatient settings, patients with suspected or confirmed mpox should be assigned to single-occupancy rooms with private bathrooms if possible. Negative-pressure isolation is not required but can be used if available. Providers should wear personal protective equipment including gowns, gloves, and eye protection. Though there is currently no epidemiologic evidence that mpox is transmitted by the airborne route, a N95 respirator is also recommended to prevent the need to change the type of mask in the event that an aerosol-producing activity is performed.28
INFECTION CONTROL AT HOME
While they are having symptoms of acute illness (eg, fever, systemic symptoms, and respiratory symptoms), patients should isolate themselves and take the following precautions to avoid transmitting the virus to household contacts:
Cover all lesions with clothing
Avoid sharing clothing, towels, face masks, and other household items such as eating utensils
Wear a well-fitting mask when in close proximity to others
If sharing a bathroom, disinfect surfaces after use
Practice frequent hand hygiene
Avoid close contact with pets, given the risk of reverse zoonosis.29
After the acute illness has passed but the skin lesions are still resolving, patients should cover all lesions with clothing and continue to perform frequent hand hygiene, avoid sharing items, and wear a mask. Full isolation is no longer required when systemic symptoms have resolved. Skin lesions should be considered infectious until all scabs have fallen off and re-epithelialization has occurred, which is generally 2 to 4 weeks in immunocompetent hosts.
HIV PROPHYLAXIS
All patients with mpox should be evaluated for HIV disease and prevention needs.
HIV-negative patients who present within 72 hours of a possible HIV exposure should receive nonoccupational postexposure prophylaxis with an approved antiretroviral regimen with appropriate baseline and follow-up HIV testing.
Patients who qualify for preexposure prophylaxis (Table 4) should be screened for HIV and started on pre-exposure prophylaxis expeditiously rather than treatment.
Indications for preexposure prophylaxis for HIV
Since most cases of mpox during the current outbreak have been sexually acquired, we would consider a diagnosis of mpox as an indication for a discussion of preexposure prophylaxis, unless a nonsexual route of acquisition can be established.
VACCINIA VACCINATION
The live, nonreplicating, modified vaccinia Ankara vaccine has been offered to individuals at high risk for mpox. Between May and October of 2022, nearly 1 million doses were administered in the United States. Vaccination consists of 2 doses, 28 days apart, given subcutaneously or intradermally. Preliminary estimates suggest that the full 2-dose series is between 60% and 80% effective.30 Though modified vaccinia Ankara is considered a live vaccine, it is replication-deficient and thus does not produce infectious virus in humans and can be given to immunocompromised individuals.
CONDOMS ARE NOT EFFECTIVE PROTECTION AGAINST MPOX
Because mpox is transmitted through direct contact with infectious lesions, barrier protection (condoms) will only impede transmission by lesions on the genitals. For groin or suprapubic lesions, barrier protection will be insufficient. Patients should be counseled that condoms, while effective for STIs such as gonorrhea and chlamydia, should not be relied on as effective mpox protection.
LESSONS LEARNED, LESSONS TO BE LEARNED
The mpox outbreak occurred at a time when public health and medical communities were still reeling from the impact of COVID-19. Mpox presented similar but also distinct challenges. While strategies for testing, vaccine distribution, and rapid information dissemination could be applied to this new challenge, mpox brought issues of stigma and homophobia to the forefront. For many, this stigmatization of mpox was reminiscent of the HIV-AIDS epidemic in the mid-1980s. While the public health response brought about some successes, there have certainly been lessons learned.
It is not yet clear what the future of mpox in non-endemic regions will be. Further clinical research is needed to characterize the epidemiology of mpox transmission including the extent to which asymptomatic individuals contribute to spread, and the risk for reverse zoonosis that could result in establishment of an animal reservoir in nonendemic regions. In addition, clinical trials are needed, designed to elucidate the optimal treatment strategies for the range of mild to severe disease. Finally, ensuring equitable access to mpox vaccination and treatments, not just in the United States but in developing countries through global assistance programs, will decrease the risk of re-emergence.
DISCLOSURES
Dr. Isaacs has disclosed contributing medical chapters for UpToDate. The other author reports no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Footnotes
Disclaimer: The views expressed in this article are those of the author and do not necessarily reflect the position or policy of the University of Pennsylvania, the US Department of Veterans Affairs, or the US government.
- Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.