A 21-year-old-man presented to the emergency department with increasing fatigue and psychosis symptoms. He had experienced no nausea, vomiting, diarrhea, chest pain, dyspnea, or urinary symptoms. He was not on any prescribed or over-the-counter medications or herbal supplements, and he denied smoking and alcohol consumption. Recently, he observed a 20-lb weight gain over 6 weeks.
On examination, the patient was alert and oriented, with clear lungs on auscultation. His temperature was 37.0°C (98.6°F), heart rate 60 beats per minute, respiratory rate 11 breaths per minute, oxygen saturation 98% on room air, blood pressure 153/92 mm Hg, and body mass index 34 kg/m2. Cardiovascular and gastrointestinal findings were unremarkable, and there were no focal neurologic abnormalities. Striae were noted over his abdomen and back.
Initial laboratory test results are listed in Table 1.
The patient’s initial laboratory test results
EVALUATION OF THE METABOLIC DISORDER
1. What is the most appropriate next step in investigating this patient’s acid-base disorder?
Random urine potassium
Random urine chloride
Random urine sodium
Random urine urea
Our patient’s blood gas analysis revealed a pH of 7.55 (indicating alkalemia), an elevated serum bicarbonate level of 34 mEq/L (suggesting a metabolic process), and an elevated partial pressure of carbon dioxide at 42 mm Hg (suggesting respiratory compensation). The patient’s partial pressure of carbon dioxide was within the expected range, which in metabolic alkalosis can be calculated using the following formula: 0.7 × (bicarbonate level + 20) ± 5. Our patient’s values were 0.7 × (34 + 20) = 44 ± 5, and he was diagnosed with primary metabolic alkalosis with respiratory compensation.
The urine chloride level is crucial to differentiate volume-responsive (urine chloride < 20 mmol/L) from volume-resistant (urine chloride ≥ 20 mmol/L) metabolic alkalosis.1 In cases of alkalemia, urine chloride is a more reliable indicator of intravascular volume depletion than urine sodium. The kidneys respond to the increased filtered bicarbonate load during volume depletion by excreting bicarbonate in the urine. The negatively charged bicarbonate, in maintaining electroneutrality, pulls positively charged sodium and potassium ions into the urine. Consequently, urine sodium and potassium concentrations may be elevated in the setting of vomiting or nasogastric suction, making them less accurate indicators of the patient’s volume status. In contrast, in a volume-depleted state urine chloride concentration remains low because low volume triggers activation of the renin-angiotensin aldosterone system, leading to increased reabsorption of sodium chloride in the proximal tubule. Therefore, the initial step in investigating metabolic alkalosis involves assessing spot urine chloride. Notably, urine urea plays no role in the evaluation of metabolic alkalosis.
Of note, serum sodium levels can aid in distinguishing various forms of metabolic alkalosis. Our patient’s hypokalemia, metabolic alkalosis, and serum sodium concentration in the upper normal range suggest a volume-expanded form of metabolic alkalosis. In contrast, volume-contracted forms (such as vomiting) typically present with a low-normal serum sodium concentration due to volume contraction and antidiuretic hormone stimulation.
CASE CONTINUED: URINE STUDY RESULTS
The results of the patient’s urine studies are listed in Table 2.
Urine studies
EVALUATION OF HYPOKALEMIA IN VOLUME-RESISTANT METABOLIC ALKALOSIS
2. Considering these laboratory results, what would be the most appropriate next course of action for this patient?
Check serum renin and aldosterone levels
Obtain urine diuretic screen
Seek consultation for genetic testing
Check 24-hour urine calcium
Check serum magnesium level
With a urine chloride level of 34 mmol/L, this patient had chloride-resistant (or volume-resistant) metabolic alkalosis along with hypokalemia. To assess the hypokalemia, urine electrolyte studies were needed to distinguish renal from extrarenal potassium losses. Although a random urine potassium level of 27 mmol/L may indicate renal potassium wasting, the accuracy of this measurement can be influenced by urine volume variations. This limitation can be overcome by evaluating the urine potassium-to-creatinine ratio or fractional excretion of potassium (calculated as [urinary potassium × serum creatinine] ÷ [serum potassium × urinary creatinine] × 100). The patient’s spot urine potassium-to-creatinine ratio was 43.5 mmol/g (values > 15 mmol/g indicate renal potassium loss).2 The fractional excretion of potassium was greater than 9.3%, indicative of inappropriate renal potassium wasting in the context of hypokalemia.2 A 24-hour urine collection, the most accurate measurement of urinary potassium excretion, also was consistent with renal potassium loss.2
Given the presence of chloride-resistant metabolic alkalosis, renal potassium wasting, and hypertension, the most prudent next step would be to assess the patient’s renin and aldosterone levels.
While diuretic abuse commonly induces hypokalemia through renal wasting and may lead to chloride-resistant metabolic alkalosis, patients typically exhibit hypotension or, at minimum, are not hypertensive. Consequently, a diuretic screen would not have been the most suitable next step in evaluating this patient.
Gitelman syndrome and Bartter syndrome are rare genetic disorders affecting the renal tubules and electrolyte balance. Gitelman syndrome is characterized by mutations in the SLC12A3 gene, leading to defects in the thiazide-sensitive sodium-chloride co-transporter in the distal convoluted tubule. This results in excessive salt and water loss, leading to hypokalemia, metabolic alkalosis, and hypomagnesemia.3 Bartter syndrome involves mutations in genes affecting the thick ascending limb of the loop of Henle, causing impaired sodium reabsorption. This leads to an electrolyte imbalance similar to that seen in Gitelman syndrome, but with hypercalciuria.4 Most patients with these syndromes have normal to low blood pressure, but hypertension can be observed in certain instances. Given our patient’s hypertension, the next sensible course of action was to evaluate his renin and aldosterone levels, not performing a 24-hour urine calcium assessment as part of the investigation for Gitelman or Bartter syndrome.
Genetic testing might have been necessary in subsequent stages of the diagnostic process, but would have been premature at this point.3,4
Hypomagnesemia can induce hypokalemia by its effects on the renal outer medullary potassium channel, leading to increased potassium excretion through the apical tubular membrane.5 Nevertheless, there is no association between hypomagnesemia and other metabolic abnormalities.
CASE CONTINUED: FURTHER TESTING
Further testing revealed a serum renin level less than 0.1 ng/mL per hour (0.5–4), serum aldosterone level 3.1 ng/dL (4–31), and serum magnesium level 2.0 mg/dL (1.7–2.2).
METABOLIC ALKALOSIS WITH INHIBITED SERUM ALDOSTERONE AND RENIN ACTIVITY
3. Based on the information at hand, the patient could have any of the following conditions except
Syndrome of apparent mineralocorticoid excess (SAME)
Liddle syndrome
Cushing syndrome or disease
Glucocorticoid-remediable aldosteronism
The additional investigation for this patient revealed inhibited serum aldosterone and renin activity. In Gitelman and Bartter syndromes, reduction of the extracellular fluid volume increases renin and aldosterone production, leading to hyperreninemia and secondary hyperaldosteronism.
SAME. Cortisol, the glucocorticoid synthesized by the adrenal gland, and aldosterone share a similar binding affinity for mineralocorticoid receptors in the principal cells of the cortical collecting duct. The renal enzyme 11 β-hydroxysteroid dehydrogenase (11β-HSD2) plays a crucial role in metabolizing cortisol to cortisone, preventing it from activating the mineralocorticoid receptor. In SAME, a mutation in the gene encoding 11β-HSD2 renders the enzyme ineffective.6 Consequently, physiologic levels of cortisol activate mineralocorticoid receptors, leading to chloride-resistant metabolic alkalosis, renal potassium wasting, hypertension, and suppression of renin and aldosterone. Usually, this genetic defect follows an autosomal recessive pattern, with affected individuals manifesting symptoms at a notably younger age, including failure to thrive and severe hypertension.6 Additionally, inhibition of 11β-HSD2 by substances such as licorice products6–9 and certain antifungal agents such as posaconazole and itraconazole8,9 can mimic the effects of SAME.
Liddle syndrome is an uncommon autosomal dominant disorder arising from mutations in the genes encoding the subunits of the epithelial sodium channel in the collecting tubule.10 This condition is characterized by an overactive epithelial sodium channel, resulting in excessive sodium reabsorption and potassium excretion, leading to chloride-resistant metabolic alkalosis, hypokalemia, hypertension, and low serum renin and aldosterone levels.10 Typically, patients present during childhood, often with a supportive family history.10 Due to variable penetrance, however, the clinical presentation and severity can vary widely. Diagnosis of Liddle syndrome usually requires genetic testing.10
Cushing syndrome is marked by the overproduction of cortisol, stemming from either endogenous sources such as a tumor (as seen in pituitary Cushing disease or other ectopic tumors causing Cushing syndrome) or exogenous sources such as medications.11 Elevated levels of circulating cortisol overpower the activity of 11β-HSD2, enabling cortisol to exert its mineralocorticoid effect on the collecting ducts.12 This results in sodium resorption and potassium excretion. Consequently, both Cushing disease and Cushing syndrome are associated with chloride-resistant metabolic alkalosis, hypokalemia, hypertension, and low serum renin and aldosterone levels.13
Glucocorticoid-remediable aldosteronism, also referred to as familial hyperaldosteronism type I, is an autosomal dominant condition marked by adrenocorticotropic hormone (ACTH)–sensitive aldosterone production in the zona fasciculata.14 Diagnosis often occurs in childhood, with many patients having a family history of hypertension and cerebrovascular and cardiovascular complications.14 While glucocorticoid- remedial aldosteronism is a cause of volume-resistant metabolic alkalosis, aldosterone levels are elevated in this condition, distinguishing it from the other causes being considered.14
Figure 1 shows an algorithm for conducting a workup and differential diagnosis of metabolic alkalosis.
Algorithm for conducting a workup and differential diagnosis of metabolic alkalosis.
CASE CONTINUED: HORMONE TESTING
The results of cortisol hormone studies are listed in Table 3. The 24-hour urine cortisol and serum ACTH levels were elevated. Therefore, the decision was made to proceed with the high-dose dexamethasone suppression test.
Cortisol hormone studies
Administering an 8-mg dose of dexamethasone, more than 10 times the amount of cortisol produced daily, is anticipated to suppress ACTH secretion from pituitary tumors. These tumors usually retain some sensitivity to high-dose glucocorticoid negative feedback inhibition. In contrast, nonpituitary tumors that ectopically produce ACTH lack active glucocorticoid receptors and typically do not respond to glucocorticoid negative feedback.15
The high-dose dexamethasone suppression test resulted in less than 50% suppression of serum cortisol, with a baseline of 66.7 μg/dL dropping to 45.2 μg/dL after an 8-mg dose of dexamethasone was administered. This finding suggested ectopic ACTH production.
Computed tomography with intravenous contrast of the chest, abdomen, and pelvis did not identify an ectopic source of ACTH production. Magnetic resonance imaging of the brain revealed a 5-mm pituitary adenoma (Figure 2).
Magnetic resonance imaging of the brain showed a 5-mm pituitary adenoma.
NEXT STEPS IN MANAGEMENT OF HYPERCORTISOLISM
4. Considering the updated information, what would be the most appropriate course of action?
Start treatment for depression
Inferior petrosal sinus sampling (IPSS)
Refer to neurosurgery for pituitary adenoma resection
Start empiric treatment with metyrapone
Individuals with hypercortisolism may exhibit various neuropsychological manifestations, as was observed in our patient.16 Although elevated cortisol levels can also be associated with depression, the concurrent presence of metabolic alkalosis, hypertension, and hypokalemia is highly unusual in cases of depression.17 Consequently, depression appeared less probable in our patient.
The clinical presentation, characterized by significantly elevated ACTH, less than 50% suppression of serum cortisol on the high-dose dexamethasone suppression test, profound hypokalemia, and metabolic alkalosis, appeared more consistent with ectopic Cushing syndrome. However, it is possible that the 5-mm pituitary adenoma could produce enough cortisol to cause the severe excess seen in this patient, especially considering that the computed tomography results of the chest, abdomen, and pelvis were normal.
Bilateral IPSS is the definitive test to distinguish between pituitary and ectopic ACTH–dependent Cushing syndrome.18 This diagnostic procedure measures ACTH levels in both the pituitary and peripheral venous drainage.18 A pituitary source is identified by a central-to-peripheral ACTH gradient of 2 or greater at baseline and 3 or greater after corticotropin-releasing hormone or desmopressin is administered, given the expectation of higher ACTH concentrations near the pituitary gland.18 Conversely, the absence of a petrosal-to-peripheral ACTH gradient indicates an ectopic ACTH source.18
If the presence of a functional pituitary adenoma is confirmed on IPSS, the patient should be referred to neurosurgery for adenoma resection. The prevalence of nonfunctioning pituitary adenomas in the population is estimated to be approximately 7 to 41.3 per 100,000.19
Given the presence of a pituitary adenoma and the inability to identify an ectopic source of ACTH production, we proceeded with IPSS to differentiate between pituitary and ectopic ACTH–dependent Cushing syndrome.
Metyrapone, a steroidogenesis inhibitor, is employed in the treatment of hypercortisolism. It is an adjunctive therapy to lower cortisol levels while awaiting surgery or as an initial option for patients with unresectable, occult, or metastatic disease.20 Metyrapone should not be started until all diagnostic evaluations are completed because it may influence the results of IPSS.
CASE CONTINUED
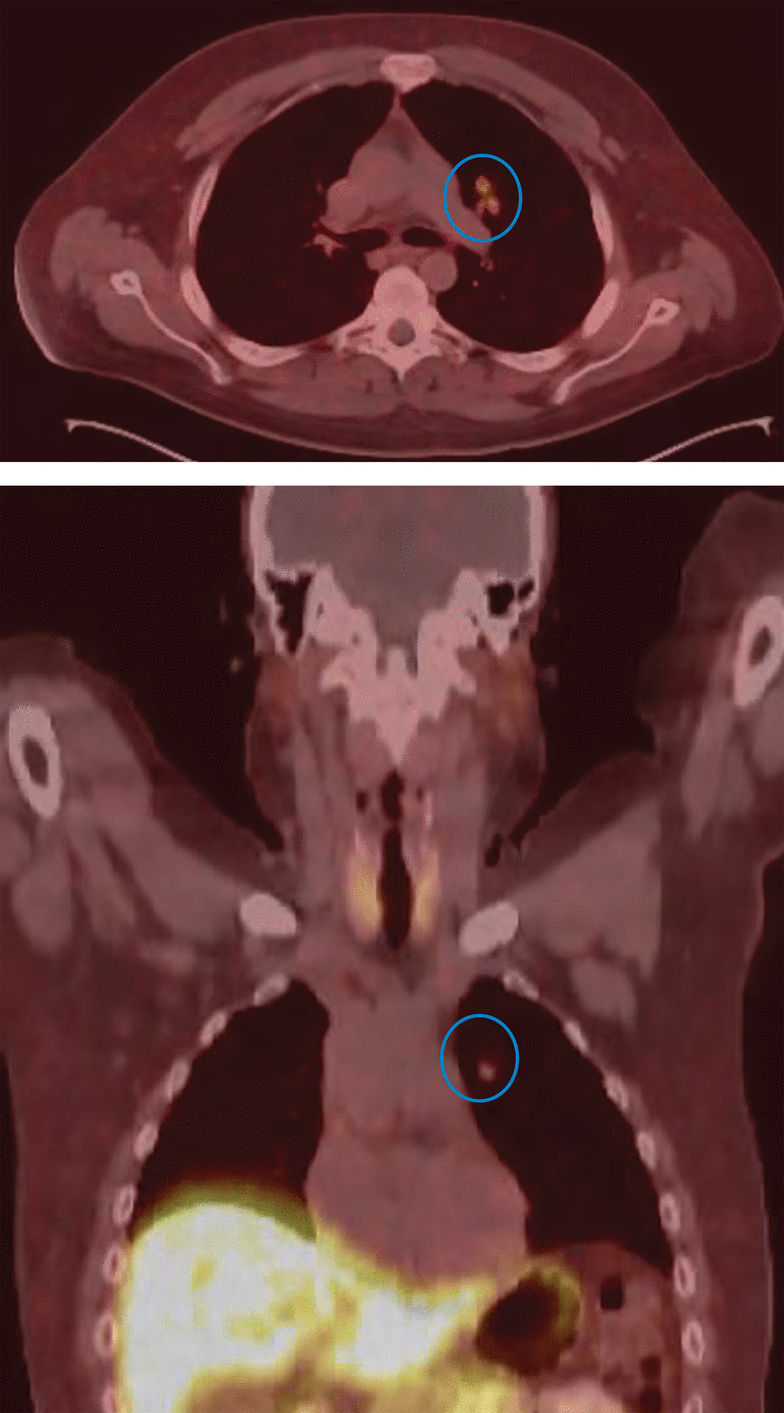
The findings from IPSS indicated a nonfunctional adenoma, supporting the diagnosis of an ectopic ACTH source as the root cause of hypercortisolism. Our patient then underwent gallium-68 dotatate positron emission tomography, revealing an anomalous tracer-avid left lung perihilar nodule with somatostatin receptor positivity (Figure 3). This finding conclusively established the diagnosis of a pulmonary carcinoid tumor.
Left lung perihilar nodule (blue circles) revealed by gallium-68 dotatate positron emission tomography.
TREATING HYPERTENSION IN ECTOPIC ADRENOCORTICOTROPIC SYNDROME
5. What is the optimal approach to managing our patient’s hypertension as he awaits resection of the lung nodule?
Losartan
Hydrochlorothiazide
Verapamil
Eplerenone
Hypertension commonly develops as a complication of ectopic ACTH syndrome, and the preferred course of action in such cases is complete resection of the ACTH-secreting tumor. Nevertheless, it is crucial to manage blood pressure while awaiting the optimal therapy.
Losartan functions as an angiotensin II receptor blocker, reducing aldosterone while elevating renin and angiotensin II levels. In Cushing syndrome, hypertension is driven by excessive cortisol rather than an abundance of aldosterone. Consequently, effectively managing blood pressure necessitates targeted therapy for hypercortisolism, often involving mineralocorticoid receptor antagonists.
Thiazides and loop diuretics should be avoided because they may exacerbate hypokalemia.
Verapamil, a nondihydropyridine calcium channel blocker, may be less efficacious in this scenario because it does not influence the mineralocorticoid pathway.
Eplerenone, a synthetic steroid and aldosterone receptor antagonist, hinders the activation of the mineralocorticoid receptor by excess cortisol.21 Eplerenone is effective in managing hypertension in Cushing syndrome and can ameliorate aldosterone-mediated metabolic alkalosis and hypokalemia.22
CASE CONCLUSION
Our patient started treatment with eplerenone and metyrapone, in addition to potassium supplements. Subsequently, he underwent resection of the left hilar lung nodule. Surgical pathology revealed a 1.2-cm typical carcinoid tumor immunohistochemically labeled with ACTH. After the tumor was removed, ACTH levels decreased, leading to resolution of both hypokalemia and hypertension. Both eplerenone and metyrapone were discontinued after surgery, and the patient no longer required potassium supplements.
Ectopic ACTH syndrome, though rare, accounts for a minority of Cushing syndrome cases. While ectopic ACTH syndrome is associated with various malignancies, neuroendocrine tumors are the most common cause.
TAKE-HOME POINTS
Begin the assessment of metabolic alkalosis by obtaining a spot urine chloride.
For patients with chloride-resistant metabolic alkalosis (urine chloride ≥ 20 mmol/L) accompanied by hypertension and low levels of serum renin and aldosterone, consider Liddle syndrome, SAME, Geller syndrome, Cushing disease and syndrome, congenital adrenal hyperplasia, and licorice use in the differential diagnosis.
Employ bilateral IPSS as the gold standard test to distinguish between pituitary and ectopic ACTH-dependent Cushing syndrome.
Recognize that ectopic ACTH syndrome, although rare, constitutes a minority of Cushing syndrome cases. It is associated with various malignancies, but neuroendocrine tumors are the most common cause of ectopic ACTH syndrome.
Choose aldosterone receptor antagonists as the primary antihypertensive for individuals with ectopic ACTH syndrome during the presurgery period.
DISCLOSURES
Dr. Hanouneh has disclosed teaching and speaking for Alexion, Astra Zeneca, BI/Lilly, and Bayer. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.