ABSTRACT
In 2023 and 2024, 3 vaccines were approved by the US Food and Drug Administration (FDA) against respiratory syncytial virus (RSV) in adults. In addition, the first long-acting RSV monoclonal antibody for infants and young children was approved. This review provides clinicians with practical guidance to navigate this new era of RSV prevention.
Palivizumab (Synagis; Sobi) and nirsevimab (Beyfortus; AstraZeneca/Sanofi) are monoclonal antibodies indicated for infants and children at high risk of RSV disease.
RSVpreF (Abrysvo; Pfizer) is a bivalent vaccine indicated for adults aged 60 and older and also for pregnant women to provide protection in their newborns.
RSVPreF3 (Arexvy; GlaxoSmithKline) is an adjuvanted vaccine approved for adults aged 60 and older and adults 50 to 59 years of age at an increased risk of RSV-associated lower respiratory tract disease.
mRNA-135 (mRESVIA; Moderna) is a messenger RNA vaccine approved for adults age 60 and older.
This review aims to provide clinicians with the tools needed to counsel patients and their families on the risks of RSV infection, the efficacy and safety of RSV immunizations, and the recommendations for their use. Clinicians can use this information to guide discussions on the risks and benefits of RSV immunization and effectively engage patients in shared clinical decision-making. This review will also summarize immunization storage and handling, cost and insurance coverage, and best practices for preventing errors.
RSV IS A MAJOR PUBLIC HEALTH PROBLEM
RSV is a leading cause of respiratory tract illness in all age groups in the United States. In healthy adults, RSV infection is typically associated with mild respiratory symptoms such as rhinorrhea, cough, sneezing, or fever.1 However, in young children and older adults, it can be a more serious illness associated with high rates of hospitalization and increased mortality.
In the United States, RSV infection leads to approximately 2.1 million outpatient visits and 58,000 to 80,000 hospitalizations per year among children less than 5 years of age, and an estimated 60,000 to 160,000 hospitalizations in adults 65 years of age or older.2 Compared with other respiratory viral illnesses, RSV infection may be less common but more severe, with higher odds of invasive mechanical ventilation or death than with influenza and COVID-19.3
With no direct-acting treatments, RSV management is typically limited to supportive care, further demonstrating the need for RSV immunizations.
ACTIVE AND PASSIVE IMMUNIZATION
Immunizations fall into 2 groups: passive immunization with monoclonal antibodies, and active immunization with vaccines.
Monoclonal antibodies. Human immunoglobulin G1 kappa antibodies with anti-RSV activity are available and can be given directly to infants and young children to confer passive immunity. These agents have not been studied or approved in adults.
Vaccines provide active immunity against RSV by inducing immune-mediated antibody development. While these vaccines provide active immunity when given to a pregnant mother, they provide passive immunity to the infant via transplacental transfer of RSV antibodies.
Development of RSV vaccines began in the 1960s, but the first formalin-inactivated vaccine failed to reach the market owing to adverse events associated with enhanced respiratory disease. Since then, additional information regarding the structure of the virus and the immunogenicity of different viral targets has been discovered.4
One of the main RSV proteins targeted by current vaccines is the fusion (F) protein, which plays a key role in fusion and attachment of the virus to the host cells. This protein exists in a prefusion and postfusion form, but the key neutralizing subunits only exist on the pre-F form of the protein. This protein is also better conserved across serotypes compared with other viral proteins, which makes it a promising target for treatment or prevention.5
Several types of RSV vaccines have been studied, including attachment protein (G-based) vaccines, purified F-protein vaccines, combination subunit vaccines, nanoparticle vaccines, and attenuated RSV vaccines.4,6 To date, only 3 vaccines have made it to market.7
The current vaccines and RSV monoclonal antibodies use the F-protein pathway to protect against RSV serotypes A and B. Table 1 summarizes the US Food and Drug Administration (FDA)-approved RSV immunizations.
Immunizations for respiratory syncytial virus (RSV)
EFFICACY AND SAFETY OF RSV MONOCLONAL ANTIBODIES
Palivizumab
Palivizumab (Synagis; Sobi) was approved in 1998 for infants and young children at high risk.
Efficacy. The approval was based on the results of the Impact-RSV trial,8 which included 1,502 preterm infants and children born at or before 35 weeks’ gestation, who were randomized to receive 5 intramuscular injections of either palivizumab or placebo, once every 30 days. By 150 days, the rates of hospitalization for RSV were 4.8% with palivizumab vs 10.6% with placebo. Further studies in children with additional risk factors for severe RSV found similar reductions in RSV hospitalization.9
Safety. Rates of adverse events were very low in the palivizumab groups. The most common events reported in the Impact-RSV trial were fever (2.8%), injection-site reactions including erythema, pain, or swelling (2.7%), and nervousness (2.5%), and the rates were all comparable to those with placebo.8,10 Most of these events were mild and resolved quickly, and only 0.3% of patients needed to stop therapy.
In real-world studies, rates of adverse reactions were still low, at 0% to 7%, the most common reactions being fever, rhinitis, and pain at the injection site.10 Rare cases of anaphylaxis have been reported, less than 1 case per 100,000 patients,11 and no deaths have been reported. It is unclear whether these reactions are related to antibody development against palivizumab.12
Nirsevimab
Nirsevimab (Beyfortus; AstraZeneca/Sanofi) was approved by the FDA in 2023 for neonates and infants born during or entering their first RSV season and for children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season. The Advisory Committee on Immunization Practices (ACIP) states that nirsevimab is indicated for infants in this group whose mothers did not receive RSV vaccination during pregnancy, whose mother’s RSV vaccination status is unknown and who were born within 14 days of the mother being vaccinated, and for children at risk of severe RSV disease.
Efficacy. Combined data from clinical trials13,14 evaluating nirsevimab vs placebo in infants younger than 8 months who were born during or entering their first RSV season of life demonstrated efficacy rates of
79.0% against medically attended lower respiratory tract RSV infection within 150 days of administration
80.6% against lower respiratory tract RSV infection requiring hospitalization
90.0% against intensive care unit admission for lower respiratory tract RSV infection.
Safety. Within 360 days after administration, only 1.2% of patients receiving nirsevimab experienced an adverse event, most (97%) of which were mild to moderate. Reactions that were more common with nirsevimab than with placebo were rash (0.9%) and injection-site reactions (0.3%), which were also the most common reactions across studies. No anaphylactic reactions were reported in the clinical trials, and the rates of serious adverse events were comparable in the placebo and palivizumab groups in each study.13,14
EFFICACY AND SAFETY OF RSV VACCINES
RSVpreF (Abrysvo; Pfizer)
RSVpreF is a recombinant antigen based on stabilized prefusion (preF) RSV F protein, derived from both the RSV-A and RSV-B subtypes. It was approved in 2023 for adults 60 years and older and also for pregnant women.
When compared with placebo in adults aged 60 or older, RSVpreF recipients had an 88.9% lower rate of RSV lower respiratory tract infection with at least 3 signs or symptoms in the first RSV season following vaccination. Preliminary data showed a 78.6% reduction partially through the second RSV season, without revaccination.15,16
In phase 3 trials, the most common reactions were fatigue (16%), headache (13%), and pain at the injection site (11%), which occurred more frequently in vaccine recipients than in those who received placebo.17 Grade 3 (severe) reactions were uncommon, occurring in less than 1% of vaccine recipients. Atrial fibrillation events were reported in 10 vaccine recipients compared with 4 placebo recipients in the 30 days following vaccination.15 There were 3 serious inflammatory neurologic events reported among 20,255 vaccine recipients, 2 cases of Guillain-Barré syndrome or Guillain-Barré variant that occurred within 14 days of vaccination, and 1 case of undifferentiated motor-sensory axonal polyneuropathy that occurred within 42 days of vaccination.15,18
In trials in pregnant women, RSVpreF was given to mothers at 24 to 36 weeks’ gestation with the goal of preventing RSV in their infants through their first 180 days of life. Efficacy rates in infants born to vaccinated mothers were
81.8% for preventing medically attended severe RSV-associated lower respiratory tract illness within 90 days after birth
69.4% for preventing medically attended severe RSV-associated lower respiratory tract illness within 180 days after birth
51.3% for preventing medically attended RSV-associated lower respiratory tract infection within 180 days after birth
56.8% for preventing RSV-associated hospitalization within 180 days after birth.19
Adverse event rates in the mothers were higher in the vaccine group than in the placebo group, with injection-site pain (41% vs 10%), myalgia (27% vs 17%), and headache (31% vs 28%) most commonly reported.19 Rates of serious or severe adverse events and events of special interest were similar between the vaccine and placebo recipients. Adverse event rates within 1 month after birth were similar between infants born to mothers that received placebo vs vaccine.19
Notably, the rate of preterm birth was higher in the vaccine group than in the placebo group, 5.7% vs 4.7%. The rate of preterm birth was slightly lower when analysis was limited to women who received vaccine or placebo between 32 and 36 weeks of gestation, 4.2% vs 3.7%.20 Based on this assessment, the FDA and the ACIP concluded that the benefit of maternal vaccination outweighs the potential risk of preterm births when given between 32- and 36-weeks’ gestation. Per FDA request, additional studies evaluating the safety of RSV maternal immunization are ongoing.
RSVPreF3 (Arexvy; GlaxoSmithKline)
The RSVPreF3 adjuvanted vaccine was approved in 2023 for use in adults 60 years and older for the prevention of symptomatic RSV lower respiratory tract infection.21
Efficacy. Clinical trials report an 82.6% reduction in symptomatic RSV lower respiratory tract infections in the first season following vaccination and a 56.1% reduction through the second RSV season, without revaccination.15,22
Safety. The most common reactions were injection-site pain (61%), fatigue (34%), myalgia (29%), and headache (27%).15,22 Despite overall higher rates of adverse events in vaccine recipients compared with placebo, 33% vs 17.8%, the rate of grade 3 events was low (4%).15 There were 10 atrial fibrillation events reported in vaccine recipients compared with 4 in placebo recipients in the 30 days following vaccination. There were 3 serious inflammatory neurologic events reported among 17,922 vaccine recipients, 1 case of Guillain-Barré syndrome occurring 9 days after vaccination, and 1 fatal and 1 nonfatal case of acute disseminated encephalomyelitis, which occurred 7 and 22 days after vaccination. However, the diagnosis of acute disseminated encephalomyelitis was later revised in both cases: 1 case was revised to the diagnosis of hypoglycemia and dementia and the other case to stroke.15
While the adverse effects of RSVpreF and RSVPreF3 cannot directly be compared, RSVPreF3 may be associated with a greater incidence of nonserious adverse events owing to the adjuvant component of the vaccine. RSVPreF3 contains the same adjuvant that is used in the recombinant zoster vaccine (Shingrix; GSK), which is well known for causing reactions. However, there are lower concentrations of the adjuvant in RSVPreF3, which may decrease the risk of adverse events.23,24 In terms of serious events, the incidence of atrial fibrillation and neuroinflammatory events, while overall low, was slightly higher in the vaccine groups compared with placebo for both vaccines. Given the rarity of these events, it remains unclear whether there is an actual association with RSV vaccination. However, these findings ultimately contributed to the ACIP’s decision to recommend RSV vaccination with shared clinical decision-making in older adults.
Expanded indication. The FDA expanded RSVPreF3’s age-based indication to include adults 50 to 59 years of age at an increased risk of RSV-associated lower respiratory tract disease in June 2024. This authorization was based on an immunogenicity study that demonstrated adults 50 to 59 years of age with chronic medical conditions achieved similar neutralizing antibody titers to RSV-A and RSV-B compared with adults 60 years of age and older.24 Despite FDA authorization, the Adult RSV Vaccine Work Group felt that there was insufficient evidence to support a recommendation to vaccinate adults 50 to 59 years of age at the ACIP June 2024 meeting.25
Use in pregnancy. RSVPreF3 is not indicated for use in pregnancy. GlaxoSmithKline also had a non-adjuvanted RSV vaccine in development for maternal immunization, but trials were stopped early due to concerns related to vaccine safety.21
mRNA-1345 (mRESVIA; Moderna)
mRNA-1345 consists of a strand of messenger RNA that encodes the RSV prefusion F protein, encapsulated in the same lipid nanoparticle that Moderna uses in its COVID-19 vaccine.26 It received FDA approval for use in people aged 60 and older in May 2024.
Efficacy. In a clinical trial in people aged 60 and older,27 at a median follow-up of 112 days, vaccine efficacy was 83.7% against RSV-associated lower respiratory tract disease with at least 2 signs or symptoms, and 82.4% against the disease with at least 3 signs or symptoms. Vaccine efficacy was 68.4% against RSV-associated acute respiratory disease.
Safety. Rates of solicited local adverse reactions were 58.7% in vaccine recipients vs 16.2% in placebo recipients, while systemic adverse reactions were reported in 47.7% vs 32.9%; most reactions were mild to moderate and transient. There were no cases of neuroinflammatory events (eg, Guillain-Barré syndrome) related to the vaccine and there was no imbalance in cardiac events, including atrial fibrillation, between vaccine and placebo recipients.
POSTMARKETING SURVEILLANCE ONGOING
The current FDA-approved RSV immunizations are safe and unlikely to cause serious adverse events. Postmarketing safety surveillance will provide additional insights on immunization safety. Clinicians should report adverse reactions to nirsevimab and palivizumab through the FDA MedWatch program online, by mail, by fax, or by calling 1-800-FDA-1088. Vaccine-related reactions, including reactions to nirsevimab or palivizumab when coadministered with a non-RSV vaccine, should be reported to the Vaccine Adverse Event Reporting System (VAERS) online, by fax, by mail, or by calling 1-800-822-7967.
ADVISORY COMMITTEE ON IMMUNIZATION PRACTICES RECOMMENDATION
The ACIP makes recommendations to the Centers for Disease Control and Prevention (CDC) on how FDA-approved vaccines should be incorporated into the US adult and pediatric immunization schedules.28 Each ACIP recommendation is constructed using the ACIP Evidence to Recommend Framework, which considers not only vaccine efficacy and safety, but factors such as feasibility, equity, and resource use.29 Based on this framework, the ACIP’s recommendations for a vaccine may differ from the FDA-approved indications.
The ACIP’s recommendations fall into 3 categories: routine, risk-based, and shared clinical decision-making (SCDM). Initially, when RSVPreF and RSVPreF3 were authorized by the FDA in the summer of 2023, the ACIP recommended adults 60 years and older be vaccinated using SCDM. In June 2024, the ACIP revised this recommendation and now recommends RSV vaccination as a routine immunization for adults 75 years and older and as a risk-based immunization for adults 60 to 74 years.30
VACCINE STORAGE AND HANDLING
Some vaccines require special storage and handling. Clinicians should ensure that all staff involved in vaccine ordering, storage, handling, and administration receive appropriate training. Resources to provide training are available through the CDC, including a comprehensive web-based training course called Building Awareness of Immunization & Vaccine.
COVID-19 vaccines need to be stored in frozen or ultra-frozen conditions, limiting the ability of some vaccine providers to stock and administer them. However, most of the RSV immunizations are stored at standard refrigerator temperatures, making them more convenient to stock in the provider office. The exception is the Moderna vaccine, which is stored in a standard vaccine freezer or can be thawed and stored refrigerated for up to 30 days. Each RSV immunization is supplied in distinctive product-specific packaging, which may help vaccine providers distinguish between products.
Palivizumab is supplied in a 50-mg/0.5-mL vial and a 100-mg/1-mL vial. The dose volume must be calculated based on each patient’s weight:
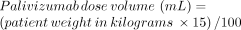
Palivizumab vials are intended to be single-dose and are preservative-free. After withdrawing the calculated dose, any remaining unused suspension should be safely discarded. Vaccine providers should not “pool” partially used vials to comprise doses. Doses greater than 1 mL in volume should be given in divided doses.12
Nirsevimab comes in a 50-mg/0.5-mL prefilled syringe and a 100-mg/1-mL prefilled syringe; each can be purchased in single-dose or 5-dose cartons. Children indicated to receive a 200-mg dose of nirsevimab in their second RSV season should receive 2 100-mg/1-mL injections. At present, the CDC recommends against using 2 50-mg/0.5-mL syringes to make up a 100-mg dose.14
RSVpreF is supplied as a kit that contains a vial of lyophilized antigen powder, a prefilled syringe of sterile water diluent, and a vial adapter. To prepare a dose, the vial of lyophilized antigen powder is reconstituted with the prefilled syringe of sterile water diluent. Vaccine providers can purchase RSVpreF in cartons that contain 1-, 5-, or 10-dose kits.16
RSVPreF3 is supplied in a 10-dose carton that contains 10 vials of lyophilized antigen powder and 10 vials of acute disseminated encephalomyelitis adjuvant suspension. To prepare a dose, 1 vial of lyophilized antigen powder is reconstituted with 1 vial of the adjuvant suspension.23 mRNA-1345 is supplied as a 0.5-mL prefilled syringe that is available for purchase in a single-dose-or 10-dose carton.
COST AND INSURANCE COVERAGE
It is important to be familiar with cost and insurance coverage when making recommendations for immunization to patients and their families. Before any RSV vaccines were approved, a survey of primary care physicians in the United States found that the most common anticipated barrier to RSV immunization would be insurance coverage and reimbursement.31 In general, public and private insurance plans are required to cover vaccines recommended in the ACIP adult and pediatric immunization schedules, including vaccines recommended with shared clinical decision-making.
An important nuance for Medicare beneficiaries is determining whether the vaccine should be given in the medical office or pharmacy setting. The COVID-19, influenza, hepatitis B, and pneumococcal vaccines are covered under Medicare Part B, meaning that they are fully covered when given in the medical office setting. Provisions from the Affordable Care Act require that these vaccines be covered in-office with no out-of-pocket expense to the patient. Other vaccines, including RSV, are covered under Medicare Part D. These vaccines are billed by the pharmacy as a prescription, so they must be administered in the pharmacy. Previously, this meant patients were subject to prescription copayments for pharmacy-based immunizations. However, the Inflation Reduction Act removed cost-sharing, so these vaccines are available to patients with a zero-dollar copay.
Adults with private insurance coverage, TriCare, or Medicaid can typically receive vaccines in the medical or pharmacy setting. While the CDC’s Bridge Access Program provides COVID-19 vaccine free of charge, programs to provide other vaccines to uninsured adults are limited.
The ACIP approved the addition of nirsevimab to the Recommended Child and Adolescent Immunization Schedule. As such, nirsevimab is covered by commercial and public insurance plans as a medical benefit with no out-of-pocket expense to the patient or their family. Nirsevimab is also provided free through the Vaccines for Children program for uninsured and underinsured children. Conversely, palivizumab is not included on the ACIP immunization schedule, nor is it provided through the Vaccines for Children Program. Instead, insurance companies require medical providers to submit for prior authorization to confirm patient eligibility for palivizumab administration.
PREVENTING IMMUNIZATION ERRORS
The Institute for Safe Medication Practices (ISMP) published a safety brief in their November 2023 newsletter advising clinicians of the risk of medication errors related to the adult and pediatric-intended RSV preventive products. Given the proximity of their release, the ISMP warned that it would be very easy for providers to mix up the product names, doses, and schedules of the RSV vaccines for adults and RSV antibodies for infants and young children.
In a January 2024 newsletter, the CDC detailed administration errors reported to the VAERS related to RSV immunizations. As of January 17, 2024, at least 25 children less than 2 years of age had erroneously received an adult RSV vaccine instead of an RSV monoclonal antibody, and 128 pregnant women had received RSVPreF3 instead of RSVpreF.
Per the CDC report, most administration errors did not result in an adverse event. Those that did occur were most often classified as nonserious, although details have not yet been published. People who experienced an administration error continue to be tracked and monitored by VAERS officials. While the exact causes of these administration errors remain unknown, resources from ISMP, CDC, and the Immunization Action Coalition provide detailed guidance on how vaccine providers can avoid the most common immunization errors. Recommended best practices are summarized in Table 2.
Strategies to prevent errors related to RSV immunizations
FUTURE DIRECTIONS
The approval of 3 new RSV immunizations in 2023 and 1 in 2024 so far marks a huge step in the advancement of RSV prevention. However, additional research is needed to expand immunization efforts to other vulnerable populations including adults less than 60 years of age with comorbid or immunocompromising conditions. The development of combined influenza, COVID-19, and RSV vaccines could also help reduce vaccine fatigue and increase vaccine uptake in the future.
Also in the pipeline is a new monoclonal antibody, clesrovimab (MK-1654; Merck); infants and children are currently being recruited for a phase 3 trial comparing this agent with palivizumab.
DISCLOSURES
Dr. DeVolld reports no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest. Dr. Rivard has disclosed serving as an advisor or review panel participant for Pfizer.
- Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.

Clicking the link below will connect you to begin the credit-claiming process for CME . After clicking on the link, scroll to the bottom of the page and click on “Complete the CME Process.” You will need your myCME login information to access this.






