ABSTRACT
An unknown number of people are born with single or multiple accessory electrical pathways between the atria and the ventricles. Although most people who have an accessory pathway never experience any problems, some show characteristic abnormalities on surface electrocardiography (the Wolff-Parkinson-White [WPW] pattern), and a minority of those with the WPW pattern experience symptoms such as palpitations, dizziness, shortness of breath, and presyncope—the WPW syndrome. The latter has the potential to lead to malignant tachyarrhythmias and even sudden cardiac death. Thus, it is imperative to detect the WPW electrocardiographic pattern, diagnose WPW syndrome early, and adequately risk stratify those at risk for serious complications.
Noninvasive tests—cardiac event monitoring, echocardiography, and exercise stress testing—can help identify those at highest risk, for whom an ablation procedure can be considered.
Electrophysiologic studies are invasive, but advances in technology have made them less risky, and many cardiologists now perform an electrophysiologic study and consider accessory pathway ablation in patients who would previously have been managed conservatively.
The management challenge lies in those with WPW pattern but no symptoms.
Tachyarrhythmias and syncope are among the most frequently evaluated problems in primary, urgent, and emergency care settings. Although their etiology is often benign, they require a thorough evaluation to assess for potentially malignant causes such as Wolff-Parkinson-White (WPW) syndrome.
WPW syndrome is a rare congenital cardiac condition in which the patient has single or multiple accessory pathways along the atrioventricular border that predispose them to potentially malignant tachyarrhythmias. It often presents with symptoms such as palpitations, dizziness, shortness of breath, presyncope, and syncope (often the symptom that prompts patients to seek medical attention), but in rare cases, the first sign or symptom is sudden cardiac death due to a malignant tachyarrhythmia.
Here we describe the pathogenesis, diagnostic strategies, general treatment guidelines, and active controversies surrounding management of WPW syndrome.
ACCESSORY PATHWAYS CAN LEAD TO MALIGNANT ARRHYTHMIAS
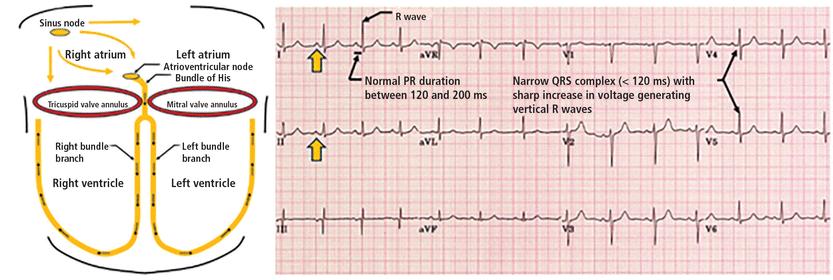
In a normal heart, electrical impulses are generated by the sinoatrial node and travel through the atrioventricular node and then down the bundle of His and the left and right bundle branches, triggering both ventricles to contract (Figure 1). The atrioventricular node is important as an electrical gatekeeper: it delays the electrical impulse long enough for the atria to contract and empty their blood into the ventricles, facilitating proper ventricular filling and helping maintain cardiac output. It also can take over as the dominant pacemaker if the sinoatrial node fails.
Left, normal conduction pathway with normal sinus rhythm generated by the sinus node and conducted through the atrioventricular node, bundle of His, and subsequently through the left and right bundle branches, leading to normal PR duration (120–200 ms), normal QRS duration (< 120 ms), and no preexcitation. Right, normal electrocardiogram with clear upright P waves (yellow arrow), normal PR interval, and narrow QRS pattern.
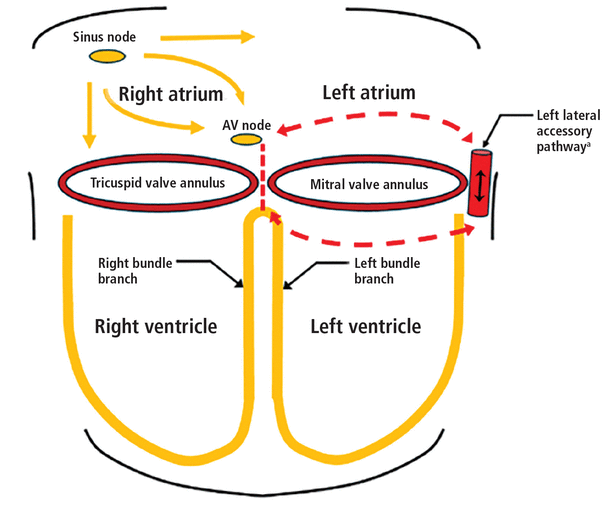
Some people, however, are born with an accessory pathway, ie, an alternate atrioventricular conduction route that bypasses both the atrioventricular node and the His system. This results in preexcitation, where the ventricles contract prematurely (Figure 2). The accessory pathway makes patients vulnerable to 2 forms of arrhythmia:
Reentrant arrhythmias such as reentrant supraventricular tachycardia, when impulses travel down the atrioventricular node and then retrograde (up) through the accessory pathway
Accelerated conduction of atrial arrhythmias, as the accessory pathway, unlike the atrioventricular node, does not delay the electrical impulse. This condition poses a significant risk in those who have atrial fibrillation by transmitting the rapid atrial rate directly to the left ventricle, potentially leading to ventricular fibrillation and sudden cardiac death.
Electrical conduction system with a left lateral accessory pathway. An accessory pathway provides an alternate atrioventricular (AV) conduction pathway, bypassing both the atrioventricular node and the His-Purkinje system, predisposing to malignant tachyarrhythmias. A left lateral accessory pathway leads to the characteristic type A Wolff-Parkinson-White pattern on electrocardiography (Figure 3).
aAccessory pathways are capable of bidirectional flow, predisposing to retrograde conduction (conduction from ventricular to atrial tissue).
DIAGNOSIS: SYMPTOMS PLUS ELECTROCARDIOGRAPHIC SIGNS
An accessory pathway can go undetected until the patient develops symptoms such as palpitations, chest pain, shortness of breath, dizziness, lightheadedness, and syncope associated with arrhythmias. However, surface electrocardiography may reveal the distinctive WPW pattern: a short PR interval (< 120 ms) and the pathognomonic finding of a delta wave, ie, a slurred upstroke of the QRS complex.
Traditionally, cardiologists used to further classify the electrocardiographic findings as 1 of 2 types (Table 1):
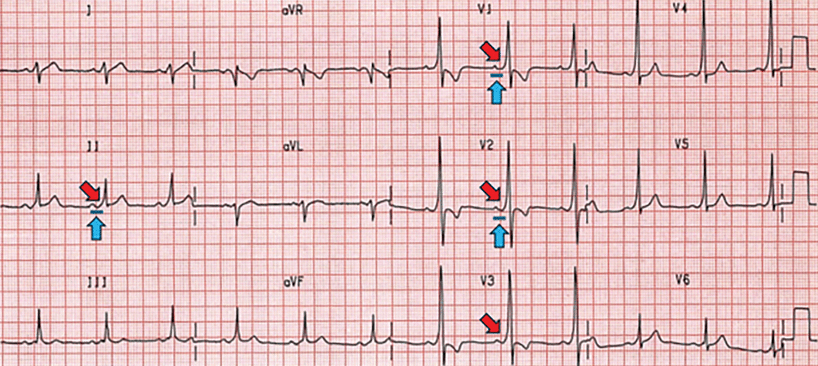
Type A (Figure 3), with delta waves in the septal leads (V1–V3), associated with an accessory pathway on the left side of the heart, or
Type B (Figure 4), with delta waves in the lateral leads (V4–V6), associated with an accessory pathway on the right.1
Type A vs type B Wolff-Parkinson-White patterns
Characteristic Wolff-Parkinson-White type A pattern, including a short PR interval (blue arrows), wide QRS, and delta waves (red arrows). Positive delta waves in V1–V3 suggest a left-sided accessory pathway (Figure 2).
Characteristic Wolff-Parkinson-White type B pattern, including a short PR interval (blue arrows), wide QRS, and delta waves (red arrows). Precordial transition after V2 suggests a right-sided accessory pathway.
But it is not so simple. Accessory pathways can exist anywhere along the atrioventricular border, resulting in patterns different from type A and type B, and even in conduction changes that are not detectable on electrocardiography. We don’t typically classify WPW accessory pathways based on type A and type B features anymore, but the classic type A and type B patterns are still commonly described in educational and reference material, as they reflect some of the most common accessory pathway locations. No specific symptoms or clinical presentations associated with a specific WPW pattern or accessory pathway location have been described.
A distinction: WPW pattern is diagnosed in patients who have no symptoms but who do have the aforementioned electrocardiographic signs, while a diagnosis of WPW syndrome means the patient has a WPW pattern and symptoms related to arrhythmias caused by the accessory pathway.
Of note, a patient can have an accessory pathway without the electrocardiographic signs, as some pathways are activated only at specific heart rates, in a specific conduction direction, or by impulses generated by the ventricle, such as premature ventricular contractions. This is important, as patients with no diagnostic electrocardiographic signs but with high clinical suspicion of having an accessory pathway may benefit from a cardiology consultation after initial evaluation in a primary, urgent, or emergency care setting.
WOLFF-PARKINSON-WHITE SYNDROME IN THE GENERAL POPULATION
WPW syndrome affects an estimated 1 to 3 individuals per 1,000 worldwide.2,3 Most people with WPW pattern have no symptoms and go on to have no clinical events related to the accessory pathway.
While most patients have normal anatomy, WPW syndrome is associated with Ebstein anomaly and hypertrophic cardiomyopathy.3,4 Approximately 10% to 34% of patients with Ebstein anomaly5,6 and 0.4% of patients with hypertrophic cardiomyopathy have WPW syndrome.7,8
In those with WPW pattern on electrocardiography who present with tachycardia, atrioventricular reentrant tachycardia is the most common arrhythmia. While atrial fibrillation is the predominant atrial arrhythmia in the general population, its incidence in patients with WPW is only 6%.8 The most severe and feared complication is sudden cardiac death, owing to the rapid atrial rates in atrial fibrillation that are transmitted directly to the ventricles by the accessory pathway, causing ventricular fibrillation. In the general population, the rate of sudden cardiac death is estimated to be 0.1% per year for patients with the WPW pattern (ie, without symptoms),9 and 0.8% per year in those with WPW syndrome (ie, with symptoms).10
MANAGEMENT STRATEGIES
Catheter ablation has a class I (strong) recommendation in patients who have symptoms and the WPW pattern (WPW syndrome).11 Catheter ablation has a high success rate (> 94%) and low complication rate (< 1%).12 Reported recurrence rates vary, but one estimate is 6.2%, and further declines are expected as techniques improve.12
The management challenge lies in those with WPW pattern but no symptoms. The goals are to alleviate symptoms related to tachyarrhythmias and to prevent the most feared complication, sudden cardiac death. While it is difficult to predict the risk of sudden cardiac death in these patients, the risk is higher in those who are male, are younger than 30 years, have a history of atrial fibrillation, have a family history of WPW syndrome, have congenital heart disease, or are in a high-risk profession such as competitive athlete, airline pilot, or professional driver, all of which should be considered when deciding on treatment.13
Noninvasive risk stratification
Noninvasive risk stratification can help guide the decision for catheter ablation.
A cardiac event monitor can be worn for as little as 24 to 48 hours and helps detect not only an abnormal heart rate and electrical impulse patterns but also preexcitation. The risk of sudden cardiac death is higher if the patient has multiple accessory pathways (which the monitor can also detect), whereas intermittent preexcitation whereby delta waves intermittently disappear even at normal heart rates suggests a lower risk profile.14,15
Echocardiography can further elucidate the risk by detecting underlying structural or congenital heart disease, as WPW syndrome is associated with Ebstein anomaly and hypertrophic cardiomyopathy.3,4
Exercise stress testing is used to find out whether the delta waves abruptly and persistently disappear with exercise, suggesting a lower risk.14,15 While this can be reassuring, it does not guarantee that atrial fibrillation will not be conducted through this pathway. There is a subset of patients who are not considered to be at high risk by noninvasive testing, but meet high-risk criteria by electrophysiologic study. Therefore, loss of preexcitation by exercise stress testing may not completely exclude an accessory pathway.15
Invasive testing (electrophysiologic study)
Electrophysiologic studies are invasive, involving intracardiac electrodes and catheters to look for and evaluate the characteristics of accessory pathways. WPW pattern or syndrome is deemed to pose a high risk for sudden cardiac death if preexcitation persists during induced atrial fibrillation or if the shortest RR interval is less than 250 ms during incremental atrial pacing, premature atrial contraction, or when in atrial fibrillation.16 while the European Society of Cardiology11 and the American Heart Association17 guidelines differ regarding methods of risk stratification, they both recommend invasive risk stratification in patients at higher risk of sudden death, including athletes and those whose sudden death could endanger other people, such as pilots and commercial drivers. Figure 5 provides our proposed management approach when a patient is found to have a WPW pattern.
Our proposed diagnostic and management guideline for patients with high clinical suspicion, Wolff-Parkinson-White (WPW) pattern, and WPW syndrome.
aHigh-risk features: male sex, age less than 30, history of atrial fibrillation, family history of WPW syndrome, congenital heart disease, competitive athlete, high-risk occupation.
bHigh risk of sudden cardiac death: multiple accessory pathways; preexcitation persists during induced atrial fibrillation; shortest RR interval < 250 ms during incremental atrial pacing, premature atrial contraction, or when in atrial fibrillation.
More electrophysiologists now than in the past may be performing electrophysiologic studies in patients with an asymptomatic WPW pattern regardless of noninvasive findings.18 The shift in strategy is believed to be due to the procedures becoming safer, with lower complication rates. Given the low risk and potential for a permanent cure via catheter ablation, there is an incentive to identify and manage WPW syndrome proactively. Additionally, recent studies have suggested that patients may experience potentially life-threatening complications of WPW syndrome even if they had no prior symptoms or “high-risk” features on noninvasive studies, providing a rationale for the current approach of evaluating patients with electrophysiologic study for risk stratification and considering ablation.19–21 This is compelling, considering the rates of electrophysiology study–related complications are reported to be as low as 1% (largely related to pneumothorax and access-site complications),22 and serious ablation-related complications are as low as 0.1% (third-degree atrioventricular block).23
Bunch et al24 in 2015 reported that long-term mortality rates for patients with WPW pattern were low and comparable to those of a control group matched by age and sex; however, patients with asymptomatic WPW pattern who underwent ablation had a lower risk of death than those who did not. This highlights the importance of careful monitoring and management of patients with WPW pattern and syndrome, given the potential for serious complications even without prior symptoms and high-risk features.
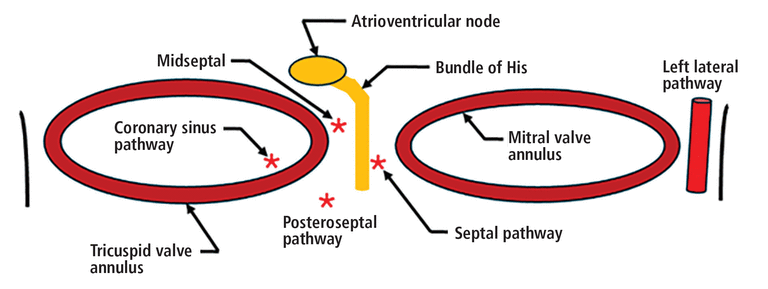
As noted above, accessory pathways can exist anywhere on the atrioventricular plane but most commonly in the left lateral mitral valve annulus (30% to 58% of reported cases), followed by the posteroseptal region (about 25% of cases), where the reported success rates of catheter ablation are excellent (> 90%). Other accessory pathway locations are associated with much lower ablation success rates, such as those surrounding the coronary sinus (50%) and in the septal (50%) and midseptal regions (73%) (Figure 6).25,26
The most reported accessory atrioventricular pathway location is the left lateral mitral valve annulus (30% to 58% of reported cases), followed by the posteroseptal region (about 25% of reported cases.25,26 The remainder of reported accessory pathway locations surround both the tricuspid annulus and the mitral valve annulus. Success rates of accessory pathway ablation vary by location, with highest success rates reported in those localized in the anterior, posterior, and lateral distribution (success rate of 90% and above), and lowest in those surrounding the coronary sinus (50%) and in the septal (50%) and midseptal distribution (73%).26
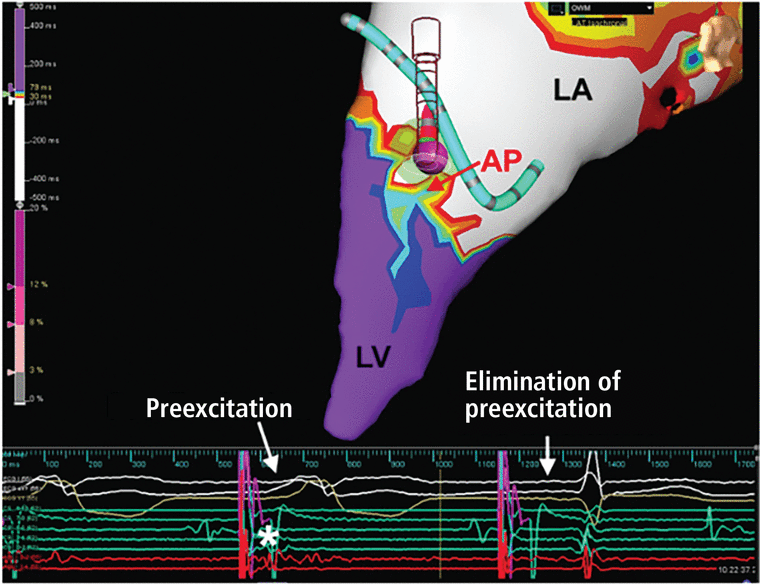
Figure 7 shows a characteristic WPW catheter ablation of a left anterolateral accessory pathway in a 21-year-old patient.
Catheter ablation of a left anterolateral accessory pathway in a 21-year-old patient. Open window mapping localized the accessory pathway (AP) to the anterolateral mitral annulus, where ablation (purple ball) eliminated the accessory pathway, with surface (white) and intracardiac electrocardiograms (red) showing loss of preexcitation and Kent potential (*) from the first to the second beat.
LA = left atrium; LV = left ventricle
In patients who remain asymptomatic and do not require ablation, observation with conservative management is an acceptable approach.11 However, given the persistence of the accessory pathway, medications that block conduction through the atrioventricular node (verapamil, diltiazem, amiodarone, digoxin, adenosine, or beta blockers) should be avoided because they can predispose to preferential conduction through the accessory pathway in the event of an atrial tachyarrhythmia, which can result in hemodynamic collapse.27
DISCLOSURES
Dr. Ho has disclosed ownership interest (stock, stock options in a publicly owned company) in Vektor Medical Inc. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2025 The Cleveland Clinic Foundation. All Rights Reserved.