ABSTRACT
The presentation of pericardial disease can be unusual, and what is assumed to be pericardial disease may not be. Knowing pericardial and mediastinal anatomy is vital for understanding these unusual presentations. This review focuses on the history, physical examination, and fundamental diagnostic testing, integrated with pericardial and mediastinal anatomy and pathophysiology.
The pericardial effusion from hypothyroidism in an elderly patient accumulates slowly and can be suspected from its classic signs and symptoms. Recognizing the radiographic appearance of a large pericardial effusion can help make the diagnosis.
When chest pain symptoms are accompanied by subcutaneous emphysema and follow prolonged Valsalva strain, pneumomediastinum should be suspected and can be confirmed by posteroanterior and lateral chest radiographs.
Because hemoglobin can, in the presence of hydrogen peroxide, act as a peroxidase, one should never use hydrogen peroxide to cleanse a draining sternotomy, which should be assumed to connect to a closed pericardial space containing blood. The result can be abrupt-onset cardiac tamponade from pneumopericardium.
Unrelenting cough can be the sole presentation of a moderate pericardial effusion.
Pericardial disease can come on suddenly or gradually. Acute pericarditis, most commonly idiopathic, presents suddenly with substernal chest pain that is worse when lying supine and with deep inspiration. In contrast, constrictive pericarditis, the result of previous acute pericarditis or another form of trauma to the pericardium (eg, violent injury, surgery, or radiation), presents gradually with dyspnea on exertion and edema.
Because the pericardium surrounds the heart, it is in the cardiologist’s bailiwick. However, the internist and the emergency physician are likely to be the first to encounter patients with pericardial disease.
A timely diagnosis can be lifesaving. When pericardial disease is suspected, the physical examination must include inspection of neck veins, palpation of the pulse, measurement of blood pressure during inspiration and expiration, auscultation of the heart and lungs, and inspection and palpation of the abdomen and lower extremities. As with all cases of chest pain and dyspnea, an electrocardiogram and a chest radiograph are necessary. When jugular venous distension, hypotension, or pulsus paradoxus is present, a significant pericardial effusion should be suspected, assessed by echocardiography, and addressed promptly.
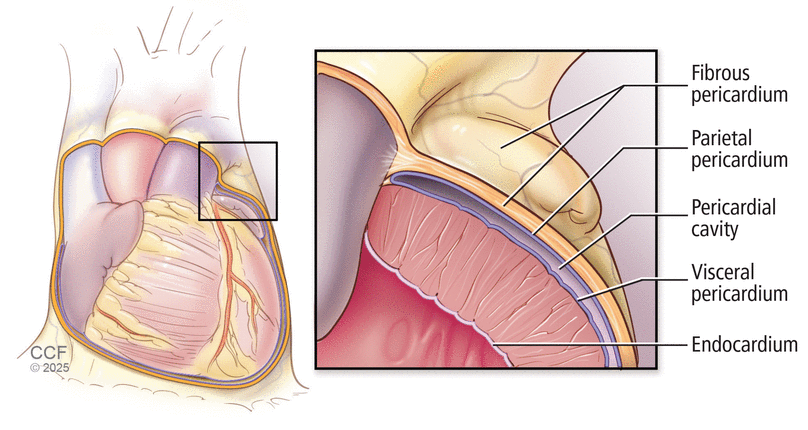
Knowledge of pericardial and mediastinal anatomy is essential. The pericardium consists of a visceral layer and a parietal layer. The visceral layer is a single serous layer that covers the surface of the heart and proximal great vessels (Figure 1), while the parietal pericardium consists of an inner serous layer, a fibrous middle layer, and the outer epicardial collagenous connective tissue (Figure 2). The pericardial space, which typically contains 15 to 50 cc of fluid, is the space between the visceral serous and parietal serous layers.1
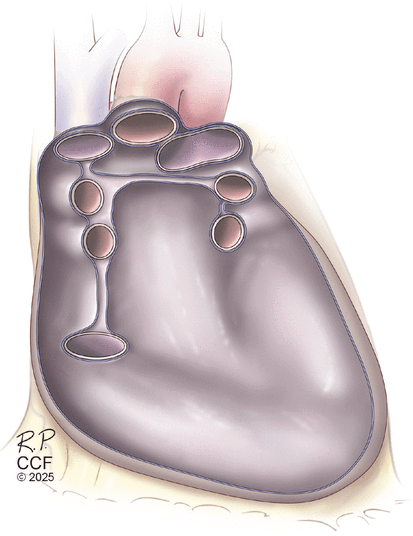
Artist’s drawing of the reflections of the anterior parietal pericardium clockwise on the superior vena cava, ascending aorta, pulmonary trunk, and diaphragm.
Artist’s drawing of posterior parietal pericardial reflections on the superior vena cava, ascending aorta, pulmonary trunk, pulmonary veins, diaphragm, and inferior vena cava.
One must learn the spectrum of pericardial disease and its mimics to formulate a differential diagnosis. You also need good physical examination skills and the ability to read a chest radiograph of the pericardium and the mediastinum.
This review presents 4 cases that can hone these diagnostic skills. Understanding the pathophysiology of each case can make the unusual more routine.
CASE 1: A 70-YEAR-OLD MAN WITH EDEMA AND FATIGUE
A 70-year-old man who had been treated by his internist with lisinopril for hypertension presented for a semiannual checkup. He complained of edema and fatigue.
On examination, his pulse was 65 per minute and regular, blood pressure 130/90 mm Hg, and weight 200 pounds (91 kg), up 10 pounds from his last visit. His arterial oxygen saturation was 95% while breathing ambient air. His voice was hoarse. His jugular veins were distended when he was sitting upright. The heart sounds were normal but distant. The right lung was clear to auscultation, but there were tubular (bronchial) breath sounds and egophony below the angle of the left scapula. There was 2+ nonpitting edema in the lower extremities. His Achilles reflexes had delayed relaxation.
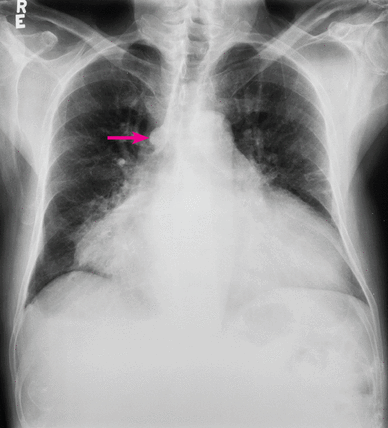
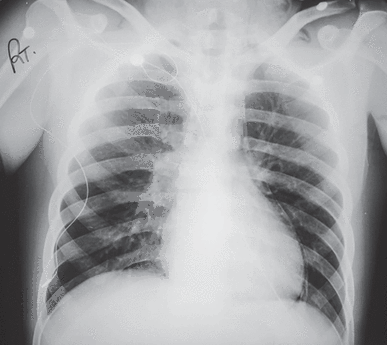
The electrocardiogram showed sinus rhythm with low QRS voltage and was otherwise normal. A posteroanterior chest radiograph (Figure 3) showed an enlarged cardiac silhouette with a broad base and a conspicuously enlarged azygos vein. There was no pleural effusion.
Posteroanterior chest radiograph in a hypothyroid patient with a large pericardial effusion and prominent azygos vein (arrow).
Reprinted from CXRs in Cardiovascular Disease, copyright 2023 by RYC, LLC; used with permission of RYP, LLC, all rights reserved.
An enlarged cardiac silhouette with a broad base is characteristic of a large pericardial effusion. Moreover, bronchial breath sounds and egophony audible beneath the left scapula, which this patient had, can be caused by a large pericardial effusion compressing the left bronchus, a phenomenon known as the Ewart sign.2 In addition, he had jugular venous distension and enlargement of the azygos vein; the azygos vein drains into the superior vena cava, and the increase in superior vena cava pressure from a large pericardial effusion can be responsible for enlargement of the azygos vein.
The results of the chest radiograph, together with the presenting symptoms and signs, prompted the internist to look for cardiac tamponade by manually checking the blood pressure during inspiration and expiration (measuring pulsus paradoxus). The systolic blood pressure during inspiration was 5 mm Hg lower than during expiration. Pulsus paradoxus less than 10 mm Hg is normal.3 Therefore, cardiac tamponade was not present.
An echocardiogram was ordered. In addition, in view of the patient’s relative bradycardia, jugular venous distension, edema, hoarseness, and Achilles reflexes, the internist ordered thyroid function tests, which confirmed the suspicion of hypothyroidism.
A cardiologist who was consulted interpreted the echocardiogram, which revealed a large pericardial effusion without signs of cardiac tamponade. As there were no signs of infection or malignancy, the internist and cardiologist agreed to treat the patient with a small oral dose of thyroid hormone (12.5 μg per day), gradually increasing the dose by 12.5 μg every 4 weeks.
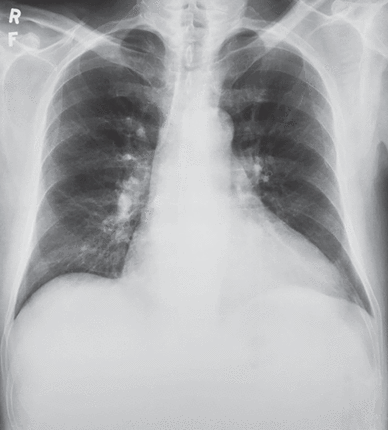
With close follow-up, the patient steadily improved. The hoarse voice, the jugular venous distension, the Ewart sign, and the edema resolved. A chest radiograph 2 months after initiation of thyroid hormone replacement showed a decrease in cardiac silhouette and disappearance of the azygos vein (Figure 4).
Posteroanterior chest radiograph of the same patient after 2 months of thyroid hormone replacement therapy.
Reprinted from CXRs in Cardiovascular Disease, copyright 2023 by RYC, LLC; used with permission of RYP, LLC, all rights reserved.
Discussion: Pericardial effusion due to hypothyroidism
In hypothyroidism, the pericardial capillaries are more permeable to albumin, and less albumin drains into the lymphatic vessels. This increases pericardial colloidal pressure and reduces the colloid osmotic pressure gradient between the pericardium and the pericardial space, which can result in fluid accumulating in the pericardial space.4
In various case series, 3% to 37% of patients with hypothyroidism developed pericardial effusions, more commonly when the hypothyroidism was severe.4,5 However, hypothyroidism is rarely the cause of pericardial effusion requiring pericardiocentesis. In 2 series of patients who underwent pericardiocentesis, hypothyroidism was the cause of the effusion in only 7 of 140 and 0 of 269 patients, respectively.6,7 In a series of 3 patients with hypothyroidism-induced pericardial effusion who underwent pericardiocentesis,5 the indication for the pericardiocentesis was hypotension— systolic blood pressure uniformly 90 mm Hg or less. The European Society of Cardiology recommends ruling out more common causes of pericardial effusion such as malignancy and bacterial infections and checking inflammatory markers.8
Pericardial effusion from hypothyroidism responds to thyroid hormone replacement, which is usually the only treatment needed.4 Of note, patients with hypothyroidism often have hypercholesterolemia, which fosters atherosclerosis. If this atherosclerosis involves the coronary arteries, it can remain silent (ie, not cause angina pectoris) because hypothyroidism slows the heart and decreases the metabolic rate. Thyroid hormone replacement therapy can unmask this hidden coronary artery disease, and for this reason, elderly patients with hypothyroidism must be initially treated with small daily oral doses of thyroid hormone and followed closely.
CASE 2: A YOUNG MAN WITH ACUTE-ONSET PLEURITIC CHEST PAIN
A 19-year-old man presented to the emergency department with severe pleuritic chest pain that started a few hours before his arrival. He was accompanied by his girlfriend, who was feeling well.
The patient grimaced with each shallow inspiration and was breathing rapidly at 20 respirations per minute. His blood pressure was 110/80 mm Hg, and his pulse was 105 per minute and regular. His arterial oxygen saturation was 98% while breathing ambient air. Auscultating the anterior chest revealed a coarse scratching sound synchronous with the heartbeat and louder during inspiration.
A presumptive diagnosis of acute pericarditis was made, and an electrocardiogram and a chest radiograph were ordered. The electrocardiogram showed sinus tachycardia and was otherwise normal. There was no ST-segment elevation.
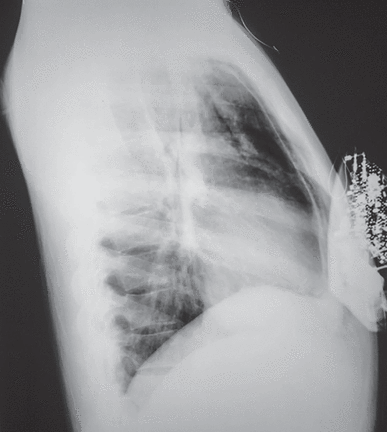
A posteroanterior chest radiograph (Figure 5) showed a normal cardiothoracic ratio (the width of the heart divided by the width of the chest is less than about 0.5), clear lungs, and no pleural effusion. Along the left heart border was a thin serosal surface separated from the heart by air. In the left supraclavicular space, there was subcutaneous emphysema. The lateral chest radiograph (Figure 6) showed air in the anterior mediastinum just behind the sternum. It was clear that there was no air surrounding the heart because the air extended well above the superior refl ections of the parietal pericardium on the ascending aorta and the superior vena cava.
Posteroanterior chest radiograph of a 19-year-old patient with mediastinal emphysema and subcutaneous emphysema.
Reprinted from CXRs in Cardiovascular Disease, copyright 2023 by RYC, LLC; used with permission of RYP, LLC, all rights reserved.
Lateral chest radiograph of same 19-year-old patient showing anterior pneumomediastinum.
Reprinted from CXRs in Cardiovascular Disease, copyright 2023 by RYC, LLC; used with permission of RYP, LLC, all rights reserved.
The patient was treated with nonnarcotic analgesics and was closely observed. The symptoms gradually resolved, and he was discharged 2 days after admission.
Discussion: Pneumomediastinum from smoking crack cocaine
The coarse sound we heard in the anterior chest is called the Hamman sign, first described by Louis Hamman in 1939.9 It sounds like 2 inflated rubber balloons rubbing against each other.
This young man developed these symptoms from mediastinal emphysema (pneumomediastinum) after he and his girlfriend were smoking crack cocaine. Trying to augment the drug’s effect, he (but not his girlfriend) performed a Valsalva strain maneuver (forced expiration against a closed glottis) after inhaling the drug.
Spontaneous pneumothorax, pneumomediastinum, and subcutaneous emphysema have been reported in patients who had been sniffing, smoking, or inhaling cocaine, heroin, 3,4-methylenedioxymethamphetamine (Ecstasy), or marijuana.10
In a series of 43 cases of cocaine-induced pneumomediastinum compiled by Alnas et al,11 93% of the patients had chest pain and 64% had subcutaneous emphysema. Symptoms subsided after a median of 24 hours, and radiologic abnormalities abated after 2 to 30 days (median of 4.5 days). Pneumothorax was present in 8 (19%) of the patients, but only 1 required a chest tube. The authors concluded that cocaine-induced pneumomediastinum is a benign condition.
Although pneumomediastinum can be spontaneous, one should identify any precipitating events like trauma, surgery, or medical procedures (eg, instrumentation) that involve the esophagus and bronchial tree. Nearly every woman performs the Valsalva maneuver during vaginal delivery, and rarely, pneumomediastinum and subcutaneous emphysema have been reported post partum.12 When a pleural effusion accompanies pneumomediastinum, mediastinal organ injury should be suspected and further evaluated.13
It is believed that pneumomediastinum results from alveolar rupture. The air tracks along the bronchovascular connective tissue planes into the mediastinum and hilum. Mediastinal air escapes into the subcutaneous tissue, resulting in subcutaneous emphysema. A prolonged Valsalva maneuver increases intra-alveolar pressure and is more likely to result in alveolar rupture.
CASE 3: A 60-YEAR-OLD MAN NEAR DEATH AFTER HEART SURGERY
A cardiologist was urgently consulted to see a 60-year-old man who was in extremis on a telemetry floor.
The patient was sitting erect and struggling to breathe. He had marked jugular venous distension, and his face was plethoric and cyanotic. The heart monitor at his bedside showed sinus tachycardia at 125 beats per minute. His fingertips were pale, and the oxygen saturation monitor would not register. His systolic blood pressure was 80 mm Hg during expiration, and Korotkoff sounds disappeared with inspiration all the way down to 0 mm Hg.
This was overt cardiac tamponade. A chest radiograph was ordered, a history was obtained, and a pericardiocentesis tray was sent for urgently.
The patient had been admitted to a local hospital because of a sternal wound infection with purulent drainage 1 week after he underwent saphenous vein graft aortocoronary bypass surgery at a referral center, with a median sternotomy. He became ill shortly after the nurse treated the sternotomy infection with topical hydrogen peroxide.
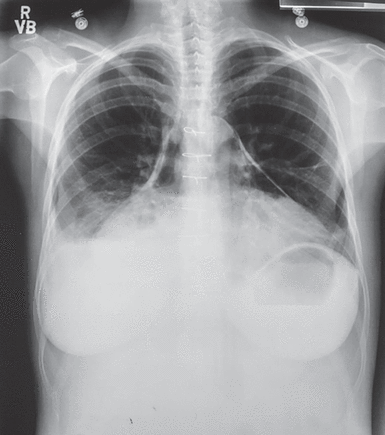
The chest radiograph showed pneumopericardium, with gas surrounding the heart in the pericardial space. Figure 7 shows a different patient with a similar problem in whom, because of pneumopericardium, the normal pericardium is less than 2 mm in thickness and the superior reflections of the parietal pericardium are within 2 to 3 cm from where the ascending aorta leaves and the superior vena cava enters the heart.
Anteroposterior chest radiograph of a female patient with pneumopericardium that demonstrates the same pericardial disease as that of the patient presented in Case 3. Because of pneumopericardium, the normal pericardium is less than 2 mm in thickness and the superior reflections of the parietal pericardium are within 2 to 3 cm from where the ascending aorta leaves and the superior vena cava enters the heart.
Reprinted from CXRs in Cardiovascular Disease, copyright 2023 by RYC, LLC; used with permission of RYP, LLC, all rights reserved.
Using sterile technique that included povidone-iodine, the cardiologist prepped the sternotomy and inserted a 16-gauge needle without a syringe 3 cm into the anterior pericardium through a rent in the sternotomy. The response was dramatic, with the sound of gas rushing through the needle and the resultant relief of symptoms. A guidewire was inserted through the needle, followed by a temporary drainage catheter.
Discussion: Oxypericardium from hydrogen peroxide
How did this gas accumulate so fast? Remember the foaming response when applying hydrogen peroxide to a bleeding skin abrasion? Hemoglobin can, in the presence of hydrogen peroxide, act as a peroxidase.14 Blood was present postoperatively in this patient’s pericardial space. The hemoglobin acted as a peroxidase with the hydrogen peroxide to release water and oxygen. The released oxygen collected in the pericardium, causing “oxypericardium,” and its sudden accumulation resulted in cardiac tamponade. The rapid accumulation of gas raised the pericardial pressure so that it exceeded central venous pressure.
Take-home point: Never cleanse a draining sternotomy with hydrogen peroxide, as it should be assumed it connects to the pericardial space, which can contain blood.
Hydrogen peroxide has been used as an antiseptic for more than 100 years. Today, it is used less often than povidone-iodine and chlorhexidine, but it is still widely available and cheap. Because it effervesces when applied to wounds, which can aid in wound debridement, hydrogen peroxide has been considered for use together with povidone-iodine and chlorhexidine for wound irrigation in orthopedic surgery.15 Due to the potential for oxygen gas formation, hydrogen peroxide should not be used in cases of dural compromise, when pressurizing medullary canals, or when irrigating smaller closed spaces, to avoid the possibility of air embolism.
CASE 4: A 67-YEAR-OLD MAN WITH COUGH
A 67-year-old man was readmitted to the hospital because of an incessant nonproductive cough 5 days after being discharged from the same hospital after saphenous vein graft aortocoronary bypass surgery and a 5-day postoperative stay. He had left the hospital with an intermittent cough and in sinus rhythm while taking aspirin, metoprolol, and a statin. He was not taking any anticoagulants. He had been prescribed these same medications without a cough for years before this operation.
A chest radiograph had been done before discharge and showed sternal wires and a small left pleural effusion, as are seen after heart surgery. However, the cardiothoracic ratio was elevated (> 0.5), and therefore an echocardiogram was ordered. This showed a small-to-moderate posterior pericardial effusion without signs of cardiac tamponade.
Now, his cough was worse. A new chest radiograph showed no change in the left pleural effusion, but the cardiothoracic ratio was further increased. A complete blood count, blood chemistries, and blood coagulation studies were normal or as expected 10 days after heart surgery. Echocardiography showed the pericardial effusion had increased to moderate in size, but again without signs of cardiac tamponade. He was afebrile, and his blood pressure was 125/80 mm Hg with less than 10 mm Hg of pulsus paradoxus. The pulse rate was 95 per minute, and he was in sinus rhythm.
Because the pericardial effusion had enlarged and the cough was worse, his physicians decided to perform a pericardiocentesis. With echocardiographic guidance the pericardial effusion was drained percutaneously, yielding 300 mL of serosanguinous fluid. Pericardial fluid analysis revealed no sign of infection or malignancy. The cough resolved.
Discussion: Cough due to pericardial effusion
Cough ascribed to a pericardial effusion was first reported by Hancock in 1983.16 Hancock’s patient’s pericardial effusion was due to idiopathic acute pericarditis. A case of cough due to a pericardial effusion resulting from radiofrequency catheter ablation for atrial fibrillation was reported by Fong et al17 in 2018. When this pericardial effusion was drained, the cough resolved.
Perhaps in cases like these the cough is due to the enlarged heart from the pericardial effusion extrinsically compressing the left bronchus—an extreme example of Ewart sign that might result in left lung collapse.18
Imazio and Adler19 note that classic symptoms of pericardial effusion include dyspnea on exertion progressing to orthopnea, chest pain, and fullness. Additional occasional symptoms due to local compression may include nausea (from compression of the diaphragm), dysphagia (from a compressed esophagus), hoarseness (from a compressed recurrent laryngeal nerve), and hiccups (from a compressed phrenic nerve). Nonspecific symptoms also include cough, weakness, fatigue, anorexia, and palpitations and reflect the compressive effect of the pericardial fluid on contiguous anatomic structures or reduced blood pressure and secondary sinus tachycardia. When pericardial effusion drainage eliminates left bronchial compression, this explanation for resolution of the cough is plausible.
Of note, pericardial effusion and cough can present as cough syncope. Saseedharan et al20 described the case of a 64-year-old man with a large malignant pericardial effusion who had subclinical cardiac tamponade that manifested as cough syncope. Pericardial drainage resulted in complete cessation of cough and resultant syncope. The exaggerated and prolonged increase in intrathoracic pressure during coughing and elevated intrapericardial pressure due to the large pericardial effusion combined to decrease cardiac filling and cardiac output sufficient to cause syncope.
NARROWING THE DIFFERENTIAL DIAGNOSIS
In the first 3 of our cases, the differential diagnosis could be narrowed to pericardial or mediastinal disease from the history and physical examination. Specifically, in Case 1, pericardial effusion from hypothyroidism presented with jugular venous distension; in Case 2, pneumomediastinum from smoking crack cocaine presented with pleuritic chest pain and the Hamman sound; and in Case 3, pneumopericardium from the use of hydrogen peroxide as a disinfectant in a closed space that contained blood presented with overt cardiac tamponade.
Each case was further defined by the results of an electrocardiogram and a chest radiograph. Case 4 presented with a cough that worsened and initially was suspected to be due to pulmonary disease. Although there was a small left pleural effusion and an increased cardiothoracic ratio by chest radiography, it was cardiac ultrasonography that was most helpful in quantifying the pericardial effusion, and it was the knowledge that a pericardial effusion can compress the left bronchus and cause a relentless cough that led to a therapeutic pericardiocentesis.
Echocardiography is the best technique to diagnose pericardial effusion. It was helpful in Case 1 and Case 4. Unfortunately, it takes time to perform and interpret. Point-of-care ultrasonography is an attractive diagnostic tool that has the potential to decrease length of hospital stay.21 The device can be carried in the pocket and, if the results were properly interpreted, they might have hastened the diagnosis and quantification of pericardial effusion in Case 1 and Case 4. Because ultrasound waves do not travel well through air (waves are reflected rather than transmitted), neither formal echocardiography nor point-of-care ultrasonography would have been beneficial in Case 2 or Case 3. Time spent by the physician performing promptly available point-of-care ultrasonography would have been time wasted.
Taking a good history, performing a good physical examination, and knowing how to read an electrocardiogram and a chest radiograph are the best starting points for diagnosing pericardial and mediastinal disease. Knowing the natural history of the disease and its pathophysiology and integrating it with the signs, symptoms, electrocardiogram, and chest radiograph allow the physician to see through the cover to the text of the book.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2025 The Cleveland Clinic Foundation. All Rights Reserved.