ABSTRACT
Statins therapy reduces atheroma in proportion to the reduction of low-density lipoprotein cholesterol (LDL-C). Proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors are a new class of injectable human monoclonal antibodies shown to lower LDL-C when added to statin therapy. In a randomized, double-blind, placebo-controlled study, 968 patients with symptomatic coronary artery disease were treated with statins alone or combined with the PCSK9 inhibitor, evolocumab, and assessed for change in percent, total volume, and regression of coronary atheroma. Treatment with statins plus evolocumab achieved mean LDL-C levels of 36.6 mg/dL, produced atheroma regression with a mean change in percent of atheroma volume of about 1% (P < .001), and induced regression in a greater percentage of patients. The clinical benefits of LDL-C as low as 20 mg/dL shown in this trial warrant further investigation.
Statin therapy achieves regression of atherosclerosis in proportion to reductions in LDL-C.
PCSK9 inhibitors are a new class of injectable human monoclonal antibodies shown to lower LDL-C when added to statin therapy.
Treatment with statins plus the PCSK9 inhibitor, evolocumab, achieved mean LDL-C levels of 36.6 mg/dL, atheroma regression, and demonstrated clinical benefit for LDL-C as low as 20 mg/dL.
Intravascular ultrasonography (IVUS) has been used for the past 20 years to measure atheromatous plaque in patients with coronary artery disease. The total volume of atherosclerosis in a coronary artery segment can be calculated using IVUS. A rotating transducer produces an image of a single, cross-sectional slice of the artery from which the atheroma area is calculated. A motorized device is used to withdraw the catheter, obtaining a series of cross-sectional slices at 1-mm intervals. The atheroma area for each slice is summated to obtain the total volume of atherosclerosis in the artery.
IVUS has demonstrated that statins slow the progression or even induce regression of coronary atherosclerosis in proportion to the degree of reduction in low-density lipoprotein cholesterol (LDL-C).1–4 No LDL-C-lowering therapy other than statins has shown regression of atherosclerosis in a trial using IVUS. The lowest LDL-C achieved in prior trials using statins was about 60 mg/dL.1,3 While this is very low, lower levels have not previously been explored.
Proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, a new class of drugs, are injectable, fully human monoclonal antibodies that inactivate the PCSK9 protein. PCSK9 inhibitors have been shown to lower LDL-C incrementally when added to statins, achieving very low LDL-C levels.5,6 However, no data exist describing the effect of low LDL-C levels reached using PCSK9 inhibitors on the progression of atherosclerosis.
THE GLAGOV TRIAL
The Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound (GLAGOV) trial assessed the effect of PCSK9 inhibitor therapy on coronary atheroma.7 The primary end point was the change in percent atheroma volume (PAV) after treatment, and secondary end points were the change in total atheroma volume and percent of patients with atheroma regression. This randomized, double-blind, placebo-controlled study included 968 patients with symptomatic coronary artery disease and other high-risk features from 197 centers around the world. Patients had a coronary angiogram with a vessel that contained an intermediate stenosis and received statin therapy for at least 4 weeks and had LDL-C levels greater than 80 mg/dL or 60 to 80 mg/dL with additional high-risk features. Following IVUS, patients were randomized for 18 months of treatment with either a statin alone or a statin plus a monthly injection of the PCSK9 inhibitor evolocumab. At the end of treatment, IVUS was performed in the same artery that we imaged at the beginning of the study (Figure 1).
Table 1 shows the patients’ baseline demographic features and statin use. The average age of patients was 60 and almost all were on statin therapy, with most taking high levels of high-intensity statins. Baseline LDL-C was very good at 92 mg/dL to 93 mg/dL, a level that would be considered good control by contemporary standards.
Baseline patient demographics and statin use
RESULTS
LDL-C levels
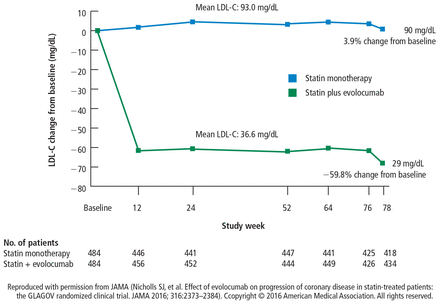
After 18 months of treatment, patients receiving statin monotherapy had a mean LDL-C of 93 mg/dL, which was essentially unchanged from the start of the study. Patients receiving statin therapy with the addition of the PCSK9 inhibitor evolocumab had a mean LDL-C of 36.6 mg/dL and a trough level of 29 mg/dL 2 weeks after dosing (Figure 2). To our knowledge, these are the lowest LDL-C levels that have ever been achieved in a major trial at the time.
Change in LDL-C for statin monotherapy and statin + evolocumab treatment arms. LDL-C = low-density lipoprotein cholesterol Reproduced with permission from JAMA (Nicholls SJ, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 2016; 316:2373–2384). Copyright © 2016 American Medical Association. All rights reserved.
Change in percent atheroma volume
With respect to the primary end point of change in PAV, patients on statin monotherapy had neither progression nor regression, and the percent change from baseline was not statistically significant (Figure 3). However, patients receiving the addition of the PCSK9 inhibitor had a statistically significant change in PAV of –0.95% (P < .001); they had less plaque at the end of the 18-month trial than at the start.
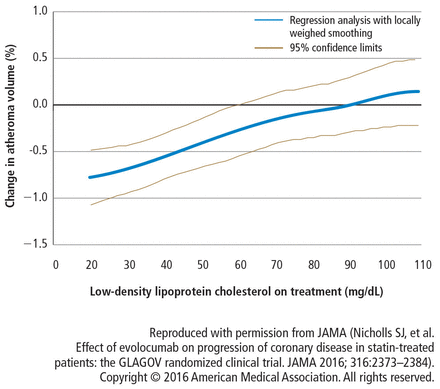
Polynomial regression analysis was used to evaluate the relationship between the achieved LDL-C levels and the rate of atheroma progression. Starting at an LDL-C of 110 mg/dL to 20 mg/dL, there was a linear relationship between lower LDL-C and less atheroma progression (Figure 4). This striking relationship was a uniform benefit across the full population and held for virtually every subgroup including by age, sex, baseline non-high-density lipoprotein cholesterol, diabetes presence or absence, and intensity of statin therapy.
Relationship between achieved low-density lipoprotein cholesterol levels and change in atheroma volume.
Total atheroma volume and percent of patients with atheroma regression
The secondary end point measuring the total atheroma volume in the coronaries showed no change in total volume of atherosclerotic plaque in the statin monotherapy group and a decrease in the statin plus evolocumab group.
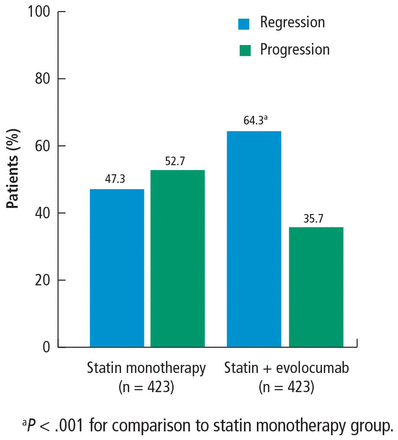
An additional secondary end point was the percent of patients with atheroma regression, defined as any decrease in total atheroma volume or PAV. The percent of patients with total atheroma volume regression was greater in the statin plus evolocumab group (61.5%) than in the monotherapy group (48.9%; P < .001). PAV regression was also greater in patients in the statin plus evolocumab group (64%) compared with patients in the statin monotherapy group (47%; P < .001) (Figure 5). It is important to note that atheroma regression cannot occur in all patients, as other factors drive atherosclerotic disease, but the high percentage of patients with manifest coronary disease experiencing regression in this study is encouraging.
Percent of patients with regression or progression of percent atheroma volume.
Based on information from reference 7.
Patients with LDL-C < 70 mg/dL
A subgroup of patients had a baseline LDL-C below 70 mg/dL, the lowest level recommended by guideline. Patients in this subgroup who received statin monotherapy remained at a mean LDL-C of 70 mg/dL whereas patients on statin plus evolocumab achieved a mean LDL-C of 24 mg/dL with a mean 2-week post-dosing trough level of 15 mg/dL, an unbelievably low level of LDL-C. In this subgroup, 81% of patients receiving statin plus evolocumab had atheroma regression, compared with 48% of patients in the statin monotherapy group. The percent of patients with atheroma regression in this subgroup of patients with low LDL-C at baseline was twice that seen in the larger study population (33% vs 17%), revealing profound levels of regression in patients treated with dual therapy.
Safety
Many people have expressed concerns about adverse effects of very low cholesterol levels. While this study was too small to evaluate morbidity and mortality, the rates of death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, and coronary vascularization trended in a favorable direction (Table 2). Essentially, no safety findings of any significance were reported in patients treated to these extremely low LDL-C levels.
Percent of patients with adverse events and safety findings
Limitations
Like all trials, this one has limitations. The population is very select: these are patients with clinically indicated angiogram, not a primary prevention population. Some study participants dropped out, which is always a limitation. And of course, this is a surrogate measure; it is a measure of disease activity, not a measure of morbidity and mortality. Morbidity and mortality data for this new class of drugs should be available in about a year, though this study suggests that those data will be favorable.
CONCLUSION
High LDL-C is universally accepted as a factor in the formation of arterial plaque and atherosclerosis. Statin therapy reduces LDL-C levels to slow or induce regression of coronary atherosclerosis in proportion to the magnitude of LDL-C reduction as measured by IVUS. However, the question of how far to reduce lipid levels has evolved over the last 4 decades. In the 1970s, a normal total cholesterol was < 300 mg/dL. More recent data that suggest optimal LDL-C levels for patients with coronary artery disease may be much lower than commonly achieved.
In this study, in patients with symptomatic coronary artery disease, treatment with statins and the addition of the PCSK9 inhibitor evolocumab achieved mean LDL-C levels of 36.6 mg/dL, produced atheroma regression with a mean change in PAV of about 1% (P < .001), induced regression in a greater percentage of patients, and showed incremental benefit for treatment of LDL-C down to as low as 20 mg/dL. The GLAGOV trial provides intriguing evidence that clinical benefits may extend to LDL-C levels as low as 20 mg/dL; however, the sample size of the trial was modest, providing limited power for safety assessments.
Since this presentation, the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial achieved a median LDL-C of 30 mg/dL and reduced risk of cardiovascular events in patients with atherosclerotic cardiovascular disease treated with evolocumab added to statin therapy.8 Additional large outcomes trials of PCSK9 inhibitors and their role in reducing LDL-C and regression of coronary atheroma and atherosclerosis are eagerly awaited.
Footnotes
This article is based on Drs. Nissen’s and Nicholls’s presentation at the Sones/ Favaloro Scientific Program, “Transforming the Delivery of Cardiovascular Care: Research and Innovation in the Heart & Vascular Institute,” held in Cleveland, OH, November 18, 2016. It was also presented at the American Association for Thoracic Surgery. The article was drafted by Cleveland Clinic Journal of Medicine and was then reviewed, revised, and approved by Drs. Nissen and Nicholls.
Dr. Nissen reported research/grant support for the Cleveland Clinic Center for Clinical Research to perform clinical trials from AbbVie, AstraZeneca, Amgen, Cerenis Therapeutics, Eli Lilly, Esperion, Pfizer, The Medicines Company, Takeda, and Orexigen Therapeutics. Dr. Nissen is involved with these multicentered clinical trials, but receives no personal remumeration for his participation. Dr. Nissen consults for many pharmaceutical companies but requires them to donate any honoraria or consulting fees directly to charity so that he receives neither income nor a tax deduction. Dr. Nicholls reported research grant support and consulting fees from Amgen, Sanofi, and Regeneron.
- Copyright © 2017 The Cleveland Clinic Foundation. All Rights Reserved.