ABSTRACT
The classification of diabetes mellitus in 2020 still starts with 2 major types, ie, type 1 and type 2, but each of these now includes a few uncommon variants. Understanding the many faces of the diabetes syndrome can make a difference in how clinicians select glucose-lowering therapy.
Variants of type 2 diabetes include monogenic forms such as maturity-onset diabetes of youth (MODY) and ketosis-prone forms such as Flatbush diabetes. In addition, when diabetes occurs with lipodystrophy, it has many features of type 2.
If patients have a Flatbush phenotype, negative autoimmune testing may help confirm the diagnosis. Although these patients need insulin at the outset, treatment can often be changed to oral glucose-lowering agents.
Lipodystrophic variants of type 2 diabetes are likely to respond to insulin sensitizers, some specifically to metreleptin.
Although type 2 diabetes has many associated genes, genetic types do not yet consistently define the specific therapeutic approaches. The exception to this is that some MODY types respond quite specifically to sulfonylureas.
The most common variant of type 1 diabetes is latent autoimmune diabetes in adults, and when this diagnosis is established either by autoimmune testing or rapid failure of several glucose-lowering therapies in sequence, insulin therapy is appropriate.
“…It is essential to realise that diabetes as commonly understood—namely the passage of sugar in the urine—is not a disease in itself. It is only a sign of disease…In brief there are several kinds of diabetes, and their outcome varies from moderate personal inconvenience to invariable fatality.”
—Anonymous, 1923.1
The statement above from nearly 100 years ago—just a few years after the discovery of insulin—is in many senses still true.2 In 2020, diabetes mellitus is still likely a syndrome with many genetic, epigenetic, and pathophysiologic abnormalities, different complication profiles, and multiple environmental influences such as infections, nutrients, exercise regimens, and the gut microbiome.3–10 The interplay among these factors is the topic of an ongoing process of discovery. Some of the discoveries help to inform the management of hyperglycemia, albeit still with many limitations.
The classification scheme in which there are 2 main types of diabetes, ie, type 1 and type 2, is still the starting point.11 Although the American Diabetes Association’s standards of care consider monogenic diabetes a separate entity,11 I believe this distinction is premature, as monogenic diabetes does not show up in the clinic as an obvious distinct entity, but rather as type 2.
However, there are variations in these 2 major types of diabetes. Pathophysiologic and genetic approaches not only provide the basis for classification schemes, but also inform the use of glucose-lowering therapy.
This article summarizes information on type 1 and type 2 diabetes mellitus, their less common subtypes, approaches to diagnosis, and implications for selecting glucose-lowering therapy. Understanding these issues does matter to the clinician.
TYPES AND BIOMARKERS OF DIABETES
Classification schemes for diabetes started to be devised more than a half century ago.12 In the 1930s, Himsworth13 infused both glucose and insulin into diabetes patients and ob served 2 distinct glucose responses: either glucose levels declined, suggesting the patient was sensitive to insulin but did not make enough of it, or glucose increased, suggesting the patient was making insulin but was resistant to it. Himsworth speculated that the latter group must be missing a factor that sensitizes people to insulin. This distinction between insulin-deficient (but sensitive) and insulin-present (but resistant) is still the framework for the current classification of diabetes mellitus.11
Assays for 2 types of soluble biomarkers helped to refine our understanding of type 1 diabetes:
Insulin and C-peptide to assess beta-cell function. Values can be low in type 1 diabetes, especially later in its course. In type 2 diabetes, insulin and C-peptide levels range from very high early in the disease process to low, but detectable, with long-standing disease.
Antibodies to islet cells and related proteins, especially glutamic acid decarboxylase. The presence of these antibodies also points to a diagnosis of type 1 diabetes.
These 2 groups of biomarkers help not only to characterize type 1 diabetes, but also to distinguish autoimmune from nonautoimmune types. They have also helped characterize subtypes of diabetes that occur in children and young adults, including:
Maturity-onset diabetes of youth (MODY), also called maturity-onset hyperglycemia of youth (MOHY)14–25
Each of these is discussed in more detail below (Table 1).8,10,11,14–19,25–41
Types of diabetes and their features
The expectation that the results of the Human Genome Project35,36 would provide greater refinement in classifying type 2 diabetes and guiding glucose-lowering regimens has not yet been fully realized.37 Dozens of genetic markers are now associated with type 2 diabetes, and many are associated with phenotypic and mechanistic components of the pathophysiology of diabetes, including insulin secretion, insulin resistance, and obesity. However, none are sufficient to subdivide type 2 diabetes in a classification scheme that would help to guide glycemic therapy.3,9,10,14,37,38
The exception is the subgroup of patients with type 2 diabetes who have MODY, in which genetic markers help characterize the appropriate pharmacotherapy.15–18 In patients who do not have genetic markers associated with response to sulfonylureas (HF-N1A, HFN4A) or the risk for complications (GCK), glucose is managed with treatment regimens generally used in type 2 diabetes mellitus.
The discussion below will only briefly mention causes of secondary diabetes and diabetes associated with lipodystrophy39–41 or hemochromatosis.42,43 The rationale for including these diseases is that each time a clinician sees a patient with diabetes, the possibility of another entity such as Cushing syndrome, acromegaly, lipodystrophy, or hemochromatosis should be considered.
Disorders associated with pancreatic damage such as cystic fibrosis and pancreatitis do not consistently result in diabetes mellitus, but when they do, insulin therapy is the best option. Since the diagnosis and treatment of pancreatic disease-associated diabetes are generally straightforward for the clinician, they will not be discussed here in detail. Gestational diabetes and rare types of neonatal diabetes will also not be discussed.
TYPE 2 DIABETES MELLITUS
The most common type of diabetes mellitus, type 2, was formerly called adult-onset diabetes or non-insulin-dependent diabetes. However, it is now known to occur also in children, and it often requires insulin therapy for glycemic control.
Type 2 diabetes is characterized by several biochemical and pathophysiologic defects associated with hyperglycemia.44 Concepts of declining insulin production not mediated by immune mechanisms and insulin resistance have been known for several decades. Additional mechanisms that have been elucidated are related to inflammation, increased hepatic glucose production, altered levels of gut hormones that regulate insulin and glucagon, and altered renal glucose thresholds. This topic has been summarized by DeFronzo.44
Many of these pathophysiologic mechanisms can now be targeted by drugs as an adjunct to diet and exercise. However, guidelines for glucose-lowering therapy take into account only general considerations of patient phenotype and comorbidities (Table 2) rather than actual pathophysiologic mechanisms.45–52
Considerations for glucose-lowering medications in type 2 diabetes mellitus
After studies of monozygotic twins and other evidence indicated that type 2 diabetes was a genetic disorder, there was hope that genetic information might be directly associated with specific pathophysiologic mechanisms involved in the development of hyperglycemia. These relationships might use genetic profiles to guide pharmacotherapy. But in spite of intriguing data demonstrating clusters of genes associated with insulin processing and signaling, as well as markers of insulin resistance, clear patterns to guide therapy are still aspirational.6,37,38
The microbiome and epigenetics are current areas of research in type 2 diabetes. However, to date, genetic and mechanistic studies have not provided clear approaches to treatment. Rather, treatment of hyperglycemia in type 2 diabetes is guided by such things as level of glycemia and comorbid conditions such as coronary heart disease, heart failure, and renal disease (Table 2). When there are marked glucose elevations, early insulin therapy should be considered because of the ability to titrate to control glucose levels. The Holy Grail of precision medicine based on genetic markers is not yet a reality.
Maturity-onset diabetes of youth
MODY is a monogenic form of nonautoimmune diabetes mellitus that often manifests in adolescents or young adults, usually before age 30.14–18 It is estimated to account for 1% to 2% of all patients with diabetes.11,15,24 Whereas MODY is widely classified as a separate type of diabetes,11 each time a clinician sees a patient with type 2 diabetes mellitus, MODY is a consideration.
Autosomal-dominant and autosomal-recessive genetic subsets of what looked like typical type 2 diabetes mellitus have been known for several decades. MODY was originally identified because of apparent autosomal-dominant patterns in families who had multiple members with non-ketosis-prone diabetes.53,54
MODY has now been characterized in several subtypes. Early genetic classifications used numbers such as MODY 1–9.16,18 Specific genetic characterization is now the standard approach. The MODY genes are broadly associated with insulin deficiency or insulin resistance. Notably, genetic subtypes associated with abnormalities of insulin secretion such as HNF1A MODY and HNF4A MODY (in adolescents and young adults) and KCNJ11 and ABCC8 (both associated with perma nent neonatal diabetes) are associated with very good glycemic responses to sulfonylureas.11,19,20,22,25 The subtype associated with abnormalities of glucokinase (GCK) does not require glucose-lowering therapy because of absence of diabetic complications with this abnormality.11,23,24 GCK mutations result in an altered glucose threshold for insulin response. Thus, patients with this abnormality usually have only mild elevations of glucose. Some patients with GCK abnormalities have normal glucose levels. Since the risk for complications is a function of the degree of hyperglycemia, and these patients have normal or only mildly elevated glucose levels, they are not at risk for microvascular complications. Thus, knowledge of these genetic subtypes helps the clinician select glucose-lowering therapy.
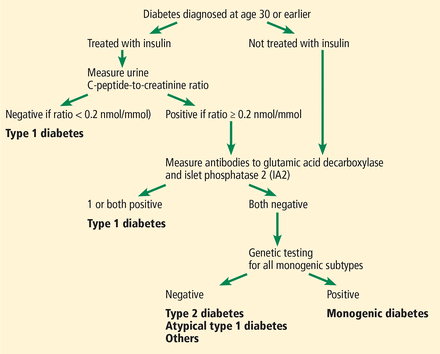
Because age of onset of MODY overlaps with that of type 1 diabetes, it is often important to distinguish MODY patients from those with type 1 diabetes to determine whether it is appropriate to treat them with drugs other than insulin. Rather than go directly to genetic testing for MODY, Shields et al17 have devised an algorithm to use in patients under age 30 (Figure 1) to distinguish MODY from type 1 diabetes. Screening begins with assessment of beta-cell function with a urinary C-peptide level. Low levels of serum C-peptide could perhaps also be used with this algorithm. Low C-peptide levels confirm type 1 diabetes. In patients with a urine C-peptide-to-creatinine ratio greater than or equal to 0.2 mmol/mg creatinine, the next step is to measure glutamic acid decarboxylase and IA2 islet cell antibodies to determine if the diabetes is autoimmune. Positive antibody tests also confirm type 1 diabetes. Patients with negative antibodies should undergo testing for MODY genes. The purpose of genetic testing is to identify MODY subtypes for which either sulfonylurea therapy or no therapy is appropriate for glycemic control.
The Using Pharmacogenetics to Improve Treatment in Early-Onset Diabetes (UNITED) biomarker screening pathway to investigate the etiology of diabetes diagnosed in patients age 30 or younger. Genetic testing is carried out in all patients who have endogenous insulin (urinary C-peptide-to-creatinine ratio ≥ 0.2 nmol/mmol) and do not have either glutamic acid decarboxylase or IA2 autoantibodies. Patients without endogenous insulin or with these antibodies are classified as having type 1 diabetes.
American Diabetes Association. Shields BM, Shepherd M, Hudson M, et al; UNITED study team. Population-based assessment of a biomarker-based screening pathway to aid diagnosis of monogenic diabetes in young-onset patients. Diabetes Care 2017; 40(8):1017–1025. Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association.
Flatbush diabetes
Flatbush diabetes was described in Afro-Caribbeans in 1994 by physicians at State University of New York Downstate based on observations in patients from the Flatbush neighborhood of Brooklyn, New York.27 Flatbush diabetes is currently considered to be on the spectrum of type 2 diabetes, although this is an issue of ongoing discussion.
At presentation, Flatbush diabetes patients have hyperglycemia with ketoacidosis. When glucose is subsequently controlled, ketosis rarely recurs. Most patients are of African descent, although Asian and Hispanic patients have also been described. These patients were originally thought to have a form of MODY, but currently known MODY genotypes are absent. These patients do not have antibodies to glutamic acid decarboxylase or to islet cells, although a few studies do report associations with human leukocyte antigens.
In a review of several reports of this type of diabetes, Lebovitz and Banerji28 note that many patients are black, male, middle-aged, overweight or moderately obese, and have a family history of type 2 diabetes.
After the ketosis at presentation has resolved, the disease looks more like type 2 diabetes. When patients present with ketoacidosis, insulin is the initial treatment of choice. When glycemic control is normal or near-normal (especially if antibody testing for glutamic acid decarboxylase or islet cell antibodies is negative), then the regimen can be changed to oral agents with approaches commonly used in type 2 diabetes.45,46
TYPE 1 DIABETES MELLITUS
Type 1 diabetes, formerly called juvenile-onset diabetes and ketosis-prone diabetes, is becoming increasingly well characterized. The pathophysiology of an autoimmune destruction of beta cells resulting in progressive insulin deficiency has been well studied over the past 40 years, and both genetic and soluble biomarker data are extensive.
Most of the time the clinical presentation is sufficient to make the diagnosis without needing measures of beta-cell function, measures of autoimmunity, or specific genetic testing. The diagnosis of type 1 diabetes clearly indicates a need for insulin replacement therapy. If there is uncertainty about the diagnosis and the corresponding need for insulin therapy, then measures of beta-cell function and islet cell antibody testing are indicated to guide treatment decisions.
The most commonly used measure of beta-cell function is the C-peptide test. Significant confounders in interpreting what may be a low C-peptide are the observations that earlier onset of type 1 diabetes is associated with lower C-peptide levels than later onset. In addition, early in the course of type 1 diabetes, C-peptide levels may still be detectable.55,56 In fact, C-peptide may be detectable for many years in patients over age 20 at diagnosis. Glucose levels should be obtained simultaneously with a C-peptide measurement to show that low C-peptide is not the result of hypoglycemia.
Latent autoimmune diabetes in adults
LADA has elements of both type 1 and type 2 diabetes.29–34 The prevalence of LADA is highly dependent on the cohort of patients under evaluation and on whether the diagnostic criteria are based on autoimmune antibodies associated with type 1 diabetes alone or on additional genetic testing in which overlap with type 2 diabetes genes is considered. Although LADA patients are often started on oral glucose-lowering agents, these agents usually do not control the glucose level for very long.
LADA should be considered in any non-obese patient who has onset of diabetes as a young adult, especially if frequent addition of oral glucose-lowering agents is needed to maintain glycemic control. This medication use pattern suggests insulinopenia, the main pathophysiologic defect in LADA.
LADA has a close kinship with type 1 diabetes because LADA patients have autoantibodies commonly associated with type 1. When LADA is suspected, glutamic acid decarboxylase and islet cell antibody testing should be performed. If these tests are positive for autoimmunity, then these patients should be switched to a regimen that includes insulin. If antibody testing is not done, but the patients have clinical features consistent with LADA—including progressive loss of glycemic control that is more rapid than commonly seen with type 2 diabetes—then insulin therapy should be initiated, even without testing for antibodies associated with type 1 diabetes.
OTHER HYPERGLYCEMIC STATES
Several other hyperglycemic states confound the classification of the diabetes syndrome. These include other endocrine disorders, medications that may increase glucose levels, and the lipodystrophies. These entities need to be considered by every physician who treats diabetes patients to avoid missing an important diagnosis. Specific therapies will not be addressed in detail except for lipodystrophy.
Endocrine disorders
Endocrine disorders including Cushing syndrome and acromegaly are often associated with hyperglycemia. If clinical features of either of these disorders are suggested by the history, physical examination, or diagnostic screens, these diagnoses should be pursued before assuming the patient has only type 2 diabetes. Hyperglycemic management follows the approach used in type 2 diabetes (Table 2).45,46
Several nonimmune pancreatic disorders are associated with diabetes. These include chronic pancreatitis and chronic recurrent acute pancreatitis (from any of multiple causes including genetic, ethanol excess, hypertriglyceridemia), cystic fibrosis, and pancreatic cancer. Usually, the associated clinical history leads to this diagnosis. Historically, glucose management includes the use of insulin.
Hemochromatosis may present only with features of diabetes, but if a family history or associated liver and cardiac disorders suggest this diagnosis, appropriate screening for iron overload and in select cases for the HFE C282Y mutation is indicated.42 Management of hemochromatosis-associated diabetes often requires insulin.
Medication-induced diabetes
Many medications can contribute to hyperglycemia, including glucocorticoids, statins, psychotropic agents, and immunomodulatory drugs.
Both glucocorticoids and immunomodulatory agents likely contribute to the entity now commonly called posttransplant diabetes. The benefits of these agents often outweigh the risks of discontinuing them simply to diminish the hyperglycemia. Tapering antirejection medications is common in posttransplant patients, and remission of diabetes may occur. However, even if there is remission of hyperglycemia, these patients should always be considered as being at increased risk for future recurrence of type 2 diabetes. Hyperglycemic management follows the approach used in type 2 diabetes (Table 2).45,46
Lipodystrophies
Lipodystrophies are uncommon, with a reported incidence of fewer than 5 cases per 1 million people.57 Nevertheless, they are important to recognize because the diagnosis may affect the selection of glucose-lowering therapy.
Lipodystrophies are broadly classified as genetic (with associated leptin deficiency) or acquired. Both genetic and acquired forms may have a pattern of general or partial loss of fat. Typical patients with lipodystrophy are described by Araujo-Vilar and Santini40 and Handelsman et al.39 In addition to hyperglycemia, lipodystrophy is often associated with moderate to markedly elevated triglycerides. The genetic disorders40 may be detected with a careful family history that suggests a genetic subtype.
Both genetic and acquired lipodystrophy require a careful physical examination to determine the extent and pattern of subcutaneous fat loss. Detecting some partial lipodystrophies may be more difficult in men than in women because men have greater muscle mass, which makes detection of loss of subcutaneous fat more difficult.
Two partial lipodystrophies deserve comment because they are commonly seen in internal medicine, endocrine, and lipid clinics. Familial partial lipodystrophy is a genetic lipodystrophy with clinical manifestations that may not occur until after puberty, so the diagnosis is often not made until adulthood.39 Human immunodeficiency virus-associated lipodystrophy is acquired and partial40 and is usually detected on examination, often in patients who have associated hypertriglyceridemia.
Treatment of hyperglycemia with lipodystrophies often parallels the treatment for type 2 diabetes and the associated dyslipidemia. However, loss of subcutaneous fat is associated with significant insulin resistance, and an insulin-sensitizing agent such as thiazolidine diones should be considered in the therapeutic regimen.39 High insulin doses may be required. The use of metreleptin is limited to patients who have the more severe lipodystrophies.39–41 Thus, a diagnosis of lipodystrophy helps to guide the clinician in the therapeutic considerations for glucose control.
Footnotes
Dr. Hoogwerf has disclosed formerly being an employee of and currently owning stock in Eli Lilly and consulting for Mannkind Corp.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.