ABSTRACT
Traditional therapies for emphysema such as bronchodilators and anti-inflammatory drugs have limited value due to permanent structural changes in the emphysematous lung that result in hyperinflation. Surgical lung volume reduction partially corrects hyperinflation by removing emphysematous lung and is an option in selected patients, but it carries a risk of morbidity and death. Valve therapy is a less-invasive option that involves bronchoscopic implantation of 1-way valves in emphysematous lung segments to allow air flow and mucus clearance in the direction of central airways. The authors review the rationale, evidence, and applications of valve therapy.
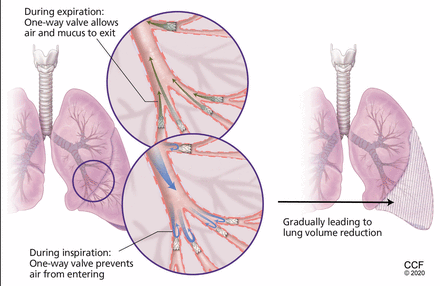
After valve placement, the 1-way flow gradually leads to selective de-aeration and collapse of treated areas, thus reducing hyperinflation.
The US Food and Drug Administration has approved valve therapy for the treatment of emphysema.
This procedure may work best for patients who have heterogeneous involvement and complete separation between affected and unaffected lobes.
Treatment of emphysema remains challenging. Standard therapies for chronic obstructive pulmonary disease (COPD) such as bronchodilators, anti-inflammatory drugs, oxygen, and pulmonary rehabilitation are of limited efficacy in the face of permanent structural changes of emphysema in the lung.
Some patients can get some relief from procedures that reduce lung volume to restore normal mechanics of the diaphragm and chest wall.1 Today, lung volume reduction is done primarily through surgery or by bronchoscopically placing 1-way valves in the airways.
In this review, we provide a clinical overview of valve therapy, the only approved bronchoscopic lung volume reduction procedure in the United States for palliation of dyspnea in selected patients with emphysema.
LUNG CHANGES IN EMPHYSEMA
Emphysema is progressive and characterized by destruction of alveolar walls distal to the terminal bronchioles, resulting in permanent enlargement of airspaces. Loss of connective tissue corresponds to loss of elastic lung recoil and reduced tethering of the small airways with consequent air trapping, hyperinflation, and collapse of small airways.
Hyperinflation increases the work of breathing by pushing the tidal volume loop to the less compliant portion of the respiratory volume-pressure curve, so that patients must generate more pressure to breathe in or out (Figure 1).2
Pressure-volume loops while breathing at rest and during exercise in a healthy individual (A) and in a patient with chronic obstructive pulmonary disease (COPD) (B). Inspiratory capacity (maximum volume of breath that can be taken in after exhalation) increases in healthy people during exercise owing to a fall in lung volume at the end of exhalation. The volume loop during normal breathing is situated in the central linear portion of the pressure-volume relationship, which means that relatively small changes in pressure produce comparatively large changes in volume. In COPD, inspiratory capacity declines due to progressive air-trapping during exercise; thus, patients have to breathe at the upper and less compliant portion of the pressure-volume relationship. This means that increasingly higher pressures must be generated for any given breath, increasing the work of breathing.
IC = inspiratory capacity; IRV = inspiratory reserve volume; P = pressure; RV = residual volume; TLC = total lung capacity; V = volume
Used with the permission of the American Thoracic Society.
Hyperinflation and air trapping are aggravated during exertion in a process called dynamic hyperinflation, caused by progressive shortening of expiratory time at high respiratory rates and consequent impaired lung emptying. At increased lung volumes, respiratory muscle fibers are shortened, creating a mechanical disadvantage in the ability to produce force. Moreover, in heterogeneous emphysema, in which emphysema is localized to a certain region of the lung, hyperinflation of the more affected areas results in compression atelectasis of other “healthier” areas, creating unfavorable ventilation-perfusion matching and poor gas exchange.1
Hyperinflation and air trapping are often seen on chest imaging and can be recognized on pulmonary function testing as increases in total lung capacity (TLC), residual volume (RV), and ratio of RV to TLC.
A BRIEF HISTORY OF LUNG VOLUME REDUCTION
In 1959, Brantigan et al3 reported that surgically removing emphysematous lung increased elastic recoil, increased radial traction on airways and restoration of a more normal configuration of the respiratory muscles. But despite subsequent improvements in surgical technique,4 lung volume reduction surgery produced varying clinical results and had a mortality rate of 4% to 17%.5
Uncertainty persisted about the risks vs benefits of this surgery, the degree and duration of clinical improvement, and patient selection criteria.
The National Emphysema Treatment Trial6 of the US Centers for Medicare and Medicaid Services and the National Heart, Lung, and Blood Institute, a multicenter randomized controlled trial, was designed to address these issues by assessing survival and exercise capacity 2 years after lung volume reduction surgery in 1,218 patients randomized (after pulmonary rehabilitation) to either undergo the procedure or continue medical therapy.
Key inclusion criteria were:
Severe emphysema: forced expiratory volume in 1 second (FEV1) < 45% of predicted, TLC > 100% of predicted, RV > 150% of predicted
Resting partial pressure of arterial carbon dioxide (Paco2) < 60 mm Hg
Resting partial pressure of arterial oxygen (Pao2) on room air > 45 mm Hg
Body mass index < 31 kg/m2 for men, < 32 kg/m2 for women
Abstinence from smoking for at least 6 months
Completion of pulmonary rehabilitation.
Exclusion criteria were significant cardiac morbidity, pulmonary hypertension (mean pulmonary artery pressure > 35 mm Hg or systolic pulmonary artery pressure > 45 mm Hg), severe functional impairment (6-minute walk distance < 140 m), chronic prednisone use, and need for high volumes of supplemental oxygen at baseline (> 6 L/minute).
At an early stage in the trial, a high-risk group with a 30-day mortality rate of 16% was identified. These patients had very severe homogeneous emphysema (FEV1 < 20% of predicted, emphysema distributed evenly throughout the lungs), or poor gas exchange (diffusion capacity < 20% of predicted). These features were added as trial exclusion criteria.6
Overall, the National Emphysema Treatment Trial showed an improvement in exercise capacity in the surgery group and no difference in mortality rate between the surgical and medical therapy groups, even after excluding the high-risk group. In subgroup analysis, patients with low baseline exercise capacity (determined by cardiopulmonary exercise testing before surgery) and upperlobe-predominant emphysema had lower mortality risk if they received surgery (risk ratio for death 0.47, P = .005). In contrast, a higher mortality rate was observed in the surgical group in the subset of patients with high baseline exercise capacity and homogeneous emphysema (risk ratio 2.06, P = .02).7 These findings were reaffirmed after a median follow-up of 5 years.8
Therefore, lung volume reduction surgery, when performed in a select group of patients with heterogeneous emphysema and low baseline exercise capacity, is a therapeutic option that prolongs survival in COPD. Patients with heterogeneous emphysema and high exercise tolerance did not derive survival benefit, although their quality-of-life scores improved.
THE NEED FOR NONSURGICAL OPTIONS
Lung volume reduction surgery has several limitations. It is associated with considerable rates of mortality (90-day mortality rate 5.2% for patients not at high risk) and morbidity (prolonged hospital stay and air leak in up to 50% of patients).7 A study performed between 2007 and 2013 showed that the in-hospital mortality rate was 5.5% and that 5.5% of patients required tracheostomy.9
While suboptimal patient selection may also have played a role in poor outcomes in this report (eg, secondary pulmonary hypertension, a relative contraindication to this surgery, was prevalent in surgery patients), alternative nonsurgical approaches to lung volume reduction are desirable.
Over the past 3 decades, several nonsurgical methods have been devised (Table 1).10-16 Among these, endobronchial valve implantation (valve therapy) is considered the most promising and is currently the only approved bronchoscopic lung volume reduction procedure in the United States.
Bronchoscopic approaches to lung volume reduction
VALVE THERAPY
Valve therapy involves implantation of 1-way valves that allow air flow and mucus clearance in the direction of central airways—out, but not in. The 1-way flow gradually leads to selective de-aeration and collapse of treated areas and reduces hyperinflation and air trapping, theoretically conducive to all the gains from lung volume reduction surgery (Figure 2). Valve therapy, unlike in lung volume reduction surgery, is performed unilaterally due to the inherent procedural risk of pneumothorax.
Valve therapy for bronchoscopic lung volume reduction involves implantation of 1-way valves to allow air flow and mucus clearance outward to central airways. The 1-way flow leads to selective de-aeration and collapse of treated areas, reducing hyperinflation and air trapping. Unlike lung volume reduction surgery, the procedure is performed unilaterally due to the inherent procedural risk of pneumothorax.
There are currently 2 valve therapy options approved in the United States: the Zephyr valve system (PulmonX, Redwood City, CA) and the Spiration valve system (Olympus, Center Valley, PA).
The VENT trial of valve therapy
The Endobronchial Valve for Emphysema Palliation Trial (VENT) was the first multicenter randomized controlled trial to assess the efficacy and safety of lung volume reduction with Zephyr endobronchial valves.17 The trial had 2 cohorts, 1 in the United States and 1 in Europe.
In the US cohort, 321 patients with severe and very severe heterogeneous emphysema were randomized in a 2:1 fashion to undergo valve placement (n = 220) or medical treatment (n = 101). Compared with medical therapy, the valve group had a modest 6.8% between-group difference in FEV1 and a 5.8% difference in 6-minute walk distance. Although statistically significant, these improvements were not considered as reaching a minimal clinically important difference. Adverse events, including pneumothorax, were more common in the valve therapy group (6.1% vs 1.2%, P = .08).17 Similar results were obtained in the European cohort.18
Given the modest benefit and substantial risk of adverse events, the US Food and Drug Administration recommended against approval of the Zephyr endobronchial valve based on the results of VENT.17
Further lessons from VENT
Post hoc analyses from VENT laid the groundwork for trials that delineated the role of endobronchial valve implantation in the treatment of emphysema.
First, improvement in lung function and 6-minute walk distance correlated with the heterogeneity of emphysema: ie, the higher the difference of emphysematous involvement between the treated lobe and neighboring lobes, the more robust the clinical improvement.17
Second, the presence of complete fissures between the lobes was associated with greater reductions in lung volume and improvement in lung function.17 This finding emphasized the importance of absence of collateral ventilation in determining success of the procedure. In essence, despite endobronchial occlusion, the treated lobe could back-fill from the neighboring lobes through collateral ventilation, thereby abrogating lung volume reduction. Absence of any interruption in the pleural lining between the lobes (so-called “fissure integrity”) was a surrogate for the absence of collateral ventilation.
Third, complete lobar occlusion was necessary for optimal results. In the VENT study, 44% of the patients had incomplete occlusion of the treated lobe, which likely lessened the benefits from the procedure.17
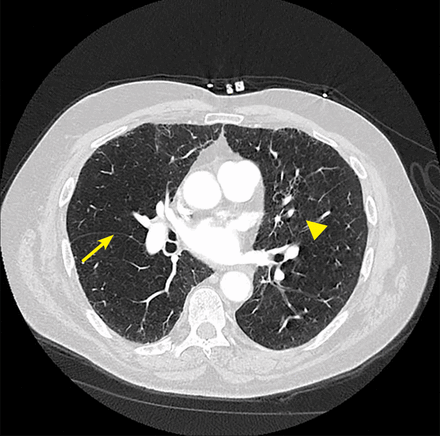
Fissure integrity (a surrogate for absence of collateral ventilation) can be assessed visually or by software analysis on high-resolution CT (Figure 3). Collateral ventilation can be directly investigated with diagnostic tools that can measure pressure and flow within the lung.
Specialized computed tomography software allows objective quantification of fissure integrity. The arrow indicates a complete fissure, and the arrowhead indicates incomplete fissure. Collateral ventilation is considered highly likely when the fissure is incomplete by > 20% across its span. This is a contraindication to valve therapy.
The Chartis Pulmonary Assessment System (PulmonX) can assess for collateral ventilation during bronchoscopy. The system consists of a balloon catheter that is used to occlude the target airway. When the balloon is inflated at the orifice of the target airway, only unidirectional (expiratory) airflow is allowed through a catheter built into the balloon. The presence of continuous expiratory airflow after balloon occlusion indicates the presence of collateral ventilation. In the absence of collateral ventilation, expiratory flow diminishes over time.
CLINICAL TRIALS OF VALVE THERAPY AFTER THE VENT STUDY
There were 7 randomized controlled trials of the clinical efficacy of valve therapy with designs that considered the experience from the VENT study (Table 2).19-25 Five of these trials used the Zephyr system,19-23 and 2 used the Spiration system.24,25 All assessed collateral ventilation during bronchoscopy using fissure analysis, the Chartis system, or both. All but 1 trial21 enrolled patients with heterogeneous emphysema in whom the treated lobe had 10% to 15% more destruction from emphysema than the neighboring lobes, based on quantitative CT analysis. One trial enrolled both heterogeneous and homogeneous emphysema patients.20
Randomized controlled trials of bronchoscopic lung volume reduction
These trials utilized clinical responder analysis as efficacy end points, defined as the proportion of patients who exhibited improvements over the minimal clinically important difference—ie, the smallest measured difference that the patient would deem significant, representing the value patients placed on the change.26 Several thresholds were used in these trials.26-29
These 7 trials recruited patients with severe to very severe COPD (mean FEV1 28% to 31% of predicted) and severe hyperinflation (mean TLC 130-144 and RV 216-277% of predicted).19-24 Compared with baseline values, patients who received valve therapy experienced lung volume reduction (mean RV reduction 0.26-0.86 L), improvement in lung function (mean increase in FEV1 8.7% to 20.9% of predicted), exertional capacity (mean intergroup difference in 6-minute walk distance 6.9-60 m) and quality-of-life scores (mean reduction of 7.2-17.3 in St. George Respiratory Questionnaire score).
Pneumothorax was the most common serious complication, occurring in 8.6% to 34.3% of patients. Some patients required the removal of valves due to recurrent pneumothorax. Two-thirds of cases occurred within the first 3 days. Consequently, patients are typically hospitalized for 3 to 5 days in anticipation of this adverse event.
Other complications included COPD exacerbations, arrhythmia, pneumonia, respiratory failure, empyema, hemoptysis, chest pain, valve expectoration or migration, bronchial trauma, and bronchial torsion. Importantly, death related to postprocedural pneumothorax was reported in some trials.
In 6 of the 7 trials, investigators and patients were not blinded to group assignment, thus introducing performance bias. In the double-blinded trial by Davey et al,19 a sham procedure was performed for the control group; this was the only study not to show a significant improvement in quality-of-life scores.19 The design of the study by Davey et al provided insight into the relative importance of performing both high-resolution CT and the Chartis procedure to assess collateral ventilation. The presence of collateral ventilation was confirmed by the Chartis system when compared with high-resolution CT, but the decision to proceed with bronchoscopic lung volume reduction was based on findings on high-resolution CT. Accordingly, 4 of the 25 patients who had intact fissures on CT were found to have collateral ventilation on assessment with the Chartis system. These patients did not experience complete lobar collapse and consequent benefits from the procedure.19 Therefore, concurrent use of the 2 assessment modalities has been advocated to increase the detection of collateral ventilation.
In the Endobronchial Valves for Emphysema Without Interlobar Collateral Ventilation (STELVIO) trial, valve replacement was needed in 17% of patients and valve removal in 22% due to recurrent pneumothorax, lack of clinical efficacy, or malpositioning.20 This finding underscores the importance of continued follow-up and personalization of care for valve therapy patients. Initial experience suggested that valve therapy worked better in patients with heterogeneous emphysema, as was seen in studies of lung volume reduction surgery.20
Both the STELVIO study and the Improving Patient Outcomes by Selective Implantation of the Zephyr EBV Study (IMPACT)21 recruited patients with homogeneous emphysema, with STELVIO using a higher threshold for air-trapping (RV > 200%) for inclusion.20,21 A meta-analysis of these data for homogeneous patients30 suggested reduction in lung volume reduction and improvement in lung function, walking distance, and quality-of- life scores comparable to that seen in patients with heterogeneous emphysema. These findings are promising for patients with homogeneous emphysema and severe hyperinflation.
In the 2 randomized controlled trials using the Spiration valve system,24,25 fissure integrity was assessed by CT. Patients were included in the trial if they had greater than 90% fissure integrity. Bronchoscopic confirmation of the absence of collateral ventilation was not required. Improvements in lung function and quality of life were similar to those in trials of the Zephyr valve. Of note, 6-minute walk distance did not improve compared with controls in the EMPROVE trial (Improving Lung Function in Severe Heterogeneous Emphysema With the Spiration Valve System).25 This was attributed to a lack of pulmonary rehabilitation in the study protocol. The pneumothorax rate was 7.6% to 28.3% in these 2 trials.24,25
PATIENT SELECTION IS KEY
Valve therapy is not for all emphysema patients. Strict adherence to clinical selection guidelines is necessary for optimal results.
Internists should consider referral for lung volume reduction for patients with severe emphysema and poor quality of life despite optimal pharmacologic treatment and pulmonary rehabilitation.
The key elements for patient selection for valve therapy are listed in Table 3.
Selection criteria for valve therapy in emphysema
Valve therapy is approved for patients with severe obstruction, hyperinflation, and air trapping and with no collateral ventilation to ensure complete lobar collapse. Collateral ventilation is assessed serially by high-resolution CT and the Chartis procedure before placement of the Zephyr valve. For the Spiration valve system, this is accomplished visually using high-resolution CT, and fissure integrity greater than 90% is required. For the Zephyr valve, if the fissure analysis indicates less than 80% completeness of the fissure adjacent to the target lobe, the likelihood of collateral ventilation is high enough that the patient should not be considered for valve therapy. For fissure integrity between 80% and 95%, patients undergo the Chartis procedure as the definitive diagnostic study for collateral ventilation. Patients with fissure integrity of 95% or greater can proceed to valve placement without the Chartis procedure.
Valves are placed in the lobe with the highest emphysema destruction score and with a greater than 10% to 15% difference compared with the neighboring lobe. These analyses are available through software systems that automatically assess fissure integrity and degree of emphysematous destruction based on x-ray attenuation.
CONCLUSION AND FUTURE DIRECTIONS
While valve therapy is a revolutionary advance in emphysema treatment, several issues deserve special attention.
First, when selection criteria are followed, only a minority of patients qualify for the procedure, principally due to lack of fissure integrity and thus the presence of collateral ventilation. For instance, in a multicenter randomized controlled trial of the Zephyr valve in patients with heterogeneous emphysema,23 of the 909 patients screened, only 190 qualified for the procedure (280 did not meet destruction score and heterogeneity criteria, 156 did not meet pulmonary function test criteria, and 65 had positive collateral ventilation, among other reasons).23
Consequently, patients should be informed about the need to have a thorough evaluation to determine candidacy. The evaluation should be holistic, exploring other options including maximizing current medical therapy, pulmonary rehabilitation, lung volume reduction surgery, and lung transplant.
Second, the impact of endobronchial valve placement on mortality rates in emphysema has not been established. None of the valve trials had death as an end point, but procedure-related deaths have been reported. The initial reports regarding mortality are encouraging31,32 but not conclusive due to the absence of an appropriate control group.
Third, valve therapy is associated with less periprocedural morbidity compared with lung volume reduction surgery. Nonetheless, surgery remains the treatment gold standard, with established benefits for selected patients. Although air leak remains very common after lung volume reduction surgery, perioperative mortality has been drastically reduced in experienced centers.33-36 The CELEB study (ISRCTN19684749) in the United Kingdom is prospectively comparing surgery vs valve placement; it completed recruitment in March 2020 and will provide important clinical insight to patient selection.37 Even so, most patients who do not qualify for valve therapy due to collateral ventilation will remain viable candidates for lung volume reduction surgery.
Fourth, the clinical trials to date have not addressed the effects of the procedure on exacerbations and on the need for less-intense pharmacotherapy. Since the procedure is associated with exacerbations of COPD, studies with longer follow-up are needed to assess the end point of COPD exacerbations, in particular.
Finally, the cost-effectiveness of the bronchoscopic procedure has not yet been established, although preliminary estimates provide optimisim.38
Valve therapy offers new hope for palliation for some patients with emphysema. A recent iteration of the Global Initiative for Chronic Obstructive Lung Disease report included valve therapy in the treatment algorithm.39 The treatment also represents an advance in personalized care for COPD. Patient selection, procedural expertise, and postprocedural care are equally important components of a successful outcome. We recommend that COPD patients undergo a thorough evaluation in specialized centers to determine the appropriate therapy for optimal outcome.
Footnotes
Dr. Machuzak has disclosed consulting for Olympus.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.