ABSTRACT
Biologic therapies have become widely used but often cause cutaneous adverse effects. The authors discuss the cutaneous adverse effects of tumor necrosis factor (TNF) alpha inhibitors, epidermal growth factor receptor (EGFR) inhibitors, small-molecule tyrosine kinase inhibitors (TKIs), and cell surface-targeted monoclonal antibodies, including how to manage these reactions and when to refer to a dermatologist.
TNF alpha inhibitors (infliximab, adalimumab, etanercept, certolizumab pegol, and golimumab) have been implicated in infusion and injection site reactions, infection, inflammatory dermatoses, and malignancy.
The most common cutaneous reaction with EGFR inhibitors (eg, gefitinib, cetuximab, erlotinib, and panitumumab) is a widespread papulopustular acneiform eruption.
Small-molecule TKIs include imatinib, dasatinib, nilotinib, ponatinib, bosutinib, sorafenib, sunitinib, pazopanib, axitinib, vandetanib, dovitinib, vemurafenib, dabrafenib, and ruxolitinib.
Commonly used monoclonal antibodies include rituximab, anakinra, tocilizumab, ipilimumab, nivolumab, pembrolizumab, and avelumab.
Biologic therapy encompasses an exponentially expanding arena of medicine. As the name implies, biologic therapies are derived from living organisms and consist largely of proteins, sugars, and nucleic acids. A classic example of an early biologic medication is insulin. These therapies have revolutionized medicine and offer targeted therapy for an increasing number of diseases, particularly in rheumatology, gastroenterology, hematologyoncology, and dermatology.
But along with these advances and the ensuing expanded use of biologic and targeted therapies have come many unique adverse effects, and some of the most commonly reported adverse effects with these new therapies are cutaneous. Cutaneous adverse effects can potentially limit the use of these agents and add cost to already expensive treatment regimens.1
It is important for physicians and other healthcare providers to be aware of these effects, have a basic understanding of how to manage patients with these reactions, and to know when to refer to a dermatologist.
This article reviews recent literature on cutaneous adverse reactions experienced with commonly prescribed biologic and targeted therapies, specifically tumor necrosis factor (TNF) alpha inhibitors, epidermal growth factor receptor (EGFR) inhibitors, small-molecule tyrosine kinase inhibitors (TKIs), and frequently used cell surface-targeted monoclonal antibodies.
TNF ALPHA INHIBITORS
TNF alpha is a proinflammatory cytokine that plays an important role in regulation of immune cells. Dysregulation of TNF alpha is involved in the pathogenesis of numerous inflammatory conditions, most notably rheumatoid arthritis, inflammatory bowel disease, psoriasis vulgaris, and psoriatic arthritis. Therefore, TNF alpha inhibitors have been successfully used to treat numerous autoimmune and inflammatory conditions.
However, these medications also have been implicated in a number of cutaneous adverse events, including infusion and injection site reactions, infection, inflammatory dermatoses, and malignancy.
Five TNF alpha inhibitors are currently available: infliximab, adalimumab, etanercept, certolizumab pegol, and golimumab (Table 1).
Cutaneous adverse effects of tumor necrosis factor alpha antagonists
Infusion reactions with infliximab
Infusion reactions associated with infliximab have been reported to occur in as many as 18% of recipients.2 These reactions may be acute (onset within minutes to hours) or delayed (days to weeks), with cutaneous manifestations of flushing, urticaria, pruritus, angioedema, and a serum sickness-like reaction.
In a Danish cohort of patients with inflammatory bowel disease receiving infliximab, infusion reactions were most strongly associated with younger patients and with episodic therapy.2
Treatment for these infusion reactions is largely supportive. Preventive measures include preinfusion treatment with oral antihistamines, acetaminophen, and occasionally intravenous steroids and slowing the rate of infusion. Adding concomitant immunosuppressive medications and avoiding drug-free intervals have also been recommended.
Injection site reactions
Injection site reactions have been reported to occur in 6% to 37% of patients receiving adalimumab, 17% to 37% of patients receiving etanercept, 6% of patients receiving golimumab, and 3.1% of those receiving certolizumab pegol.3,4
Patients can experience itching, pain, redness, irritation, bruising, or swelling at the injection site. This can be seen during the first month of treatment and can last 3 to 5 days. Absence of warmth or drainage and improvement within a few days can distinguish injection site reactions from infection.
Management of these reactions is again primarily supportive. Preventive therapies similar to those described for infusion reactions, as well as cooling pads or ice packs for symptomatic relief, may be helpful. Varying the site of injection is another useful strategy. Most of these reactions are considered moderate, and rarely do patients need to discontinue the TNF alpha inhibitor because of them.
Cutaneous infections
TNF alpha plays an important role in numerous complex immune signaling pathways, including cell proliferation, differentiation, apoptosis, macrophage activation, and morphogenesis of lymphoid tissue. Not surprisingly, inhibition of this cytokine leads to increased risk of cutaneous infection. Risk factors for increased cutaneous infections during TNF alpha inhibitor therapy include additional immunosuppressive therapy, malnutrition, age, and comorbidities such as chronic lung disease, alcoholism, organic brain disease, and diabetes mellitus.
A single-center, retrospective cohort study5 of 583 patients with inflammatory bowel disease treated with TNF alpha inhibitors (primarily infliximab) found cutaneous infections to be the most common dermatologic complication of therapy. The cumulative incidence of cutaneous infection was 1.1% at 1 year of therapy, 6.4% at 5 years, and 17.6% at 10 years; the median time to onset was 3 years. Bacterial infections (overwhelmingly staphylococcal) were the most common and manifested as folliculitis, erysipelas, cellulitis, and abscess formation. Cutaneous infection led to discontinuation of therapy in 2.9% of those affected.5 Fungal cutaneous infections, particularly with Candida species, are more common when a corticosteroid is combined with a TNF alpha inhibitor, but the exact incidence is not known.
Another large cohort study of patients treated with TNF alpha inhibitors6 also found an increased incidence of bacterial skin infections, as well as a high incidence of herpes virus skin infections. This population-based study from Spain6 found cutaneous bacterial infections occurred at an incidence of 10.4 per 1,000 patient-years, and zoster infections at an incidence of 7.2 per 1,000 patient-years. Zoster infections were found more often in those receiving infliximab and adalimumab. In addition, immunosuppressive therapy in conjunction with a TNF alpha inhibitor increased the risk of zoster dissemination and complications, including bacterial superinfection and postherpetic neuralgia.
Cutaneous infections during anti-TNF alpha therapy are rarely serious, and management should include frequent skin examinations and initiation of appropriate antibacterial or antifungal topical or oral therapy. For example, acyclovir, valacyclovir, and famciclovir can be used for acute varicella zoster virus infection.
Given the current guidelines and the incidence of herpes zoster in patients receiving TNF alpha inhibitors, clinicians should strongly consider vaccination before starting therapy.6 Safe use and efficacy of the recombinant vaccine in these individuals are not entirely clear. There are currently no contraindications to the recombinant vaccine in patients on moderate- to high-dose immunosuppressive therapy. However, data on efficacy and safety are not yet sufficient to recommend routinely giving the recombinant vaccine to patients actively treated with TNF alpha inhibitors.7
Human papillomavirus infections can cause anogenital warts and cervical dysplasia and may have an increased incidence in patients on TNF alpha inhibitors. A study of women with inflammatory bowel disease found that those receiving a TNF alpha inhibitor were more likely to have abnormal Papanicolaou smears than were controls (odds ratio [OR] 4.5) and those with inflammatory bowel disease not on TNF alpha inhibitors (OR 1.9).8
However, another study of 222 patients on TNF alpha inhibitors found that even after a mean of 31.4 months on the medication, there was no increase in detectable anogenital human papillomavirus infection or disease. Given the mixed findings, standard vaccination and screening schedules should be adhered to in this population.
Dermatitis
Numerous inflammatory and autoimmune-like cutaneous reactions have been reported during anti-TNF alpha therapy; they include psoriasis, eczema, lupus erythematosus, vasculitis, and others.
The terms psoriasiform dermatitis and psoriasis are sometimes used interchangeably when describing psoriasis-like lesions in the setting of anti-TNF alpha agents. We have therefore kept the terminology consistent with that used by the authors of the study being discussed.
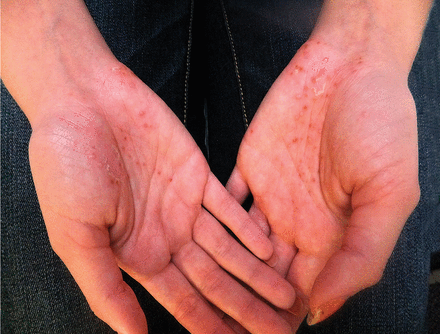
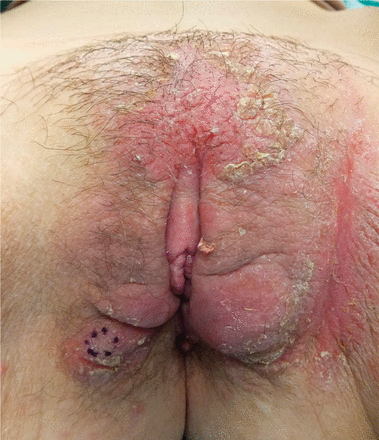
Psoriasiform lesions. A case-control study9 involving 521 patients with inflammatory bowel disease treated with TNF alpha inhibitors examined those within the cohort who developed psoriasiform skin lesions. Psoriasiform lesions were reported in 3.5% of patients and most commonly involved the palms, soles, and scalp (Figure 1). Other areas that can be affected are the intertriginous and genital skin (Figure 2).
Palmar psoriasis eruption in a patient receiving infliximab treatment for Crohn disease.
Inverse psoriasis induced by infliximab treatment for Crohn disease.
On biopsy, psoriasiform lesions have histologic features similar to those of psoriasis, and further, can resemble allergic contact dermatitis, seborrheic dermatitis, atopic dermatitis, pityriasis rubra, and lichen simplex chronicus.
One study5 found a cumulative incidence of psoriasiform dermatitis of 1.1% at 1 year, 6.75% at 5 years, and 28.9% at 10 years in a cohort of patients with inflammatory bowel disease treated with TNF alpha inhibitors. In those who developed psoriasiform dermatitis, topical therapy was required in 78% and systemic therapy (methotrexate or phototherapy) in 15.2%. Remission occurred in 20.3%, and 18.6% of patients needed to discontinue TNF alpha inhibitor therapy.
Another study10 examined 102 patients with TNF alpha inhibitor-induced psoriasis and found the most common forms to be plaquetype (49.5%) and scalp (47.5%). Palmoplantar pustulosis (whose differential diagnosis can include dyshidrotic eczema, contact dermatitis, pityriasis rubra pilaris, acquired palmoplantar keratoderma, tinea pedis, and tinea manuum) was also found in 41% of these patients. Topical medications alone improved the eruption in 63.5% of patients, with cyclosporine and methotrexate often successful when topical treatments alone failed.10 Discontinuation of the TNF alpha inhibitor was required in 10.6% of those who developed lesions.
Notably, however, lesions commonly recur if a TNF alpha inhibitor, either the same drug or a different one, is restarted after discontinuation. In a study of patients with inflammatory bowel disease treated with TNF alpha inhibitors, 9 patients had to stop the medication because of psoriasiform lesions. Three of those were retreated with a second TNF alpha inhibitor, and all had recurrence of the lesions.9 Current experience has found that there is high risk of recurrence with use of the same TNF alpha agent, and about a 50% recurrence rate if using another drug in the same class.9,10
An algorithm for treating TNF alpha inhibitor psoriasiform eruptions has been proposed and is based on severity of skin eruption and control of the underlying disease11:
If the skin eruption is mild and the underlying disease is controlled, continue the TNF alpha inhibitor and treat the eruption topically
If the skin eruption is mild but the underlying disease is not controlled, switching within the same class is reasonable
If the skin eruption is moderate to severe and the underlying disease is controlled, switching within the same class is reasonable
If the skin eruption is moderate to severe and the disease is not well controlled, discontinuing TNF alpha inhibitors altogether is warranted.
Other forms of dermatoses reported in association with TNF alpha inhibitors tend to occur less often than psoriasiform eruptions and include eczema, leukocytoclastic vasculitis, lupus erythematosus, and granulomatous dermatitis. Eczematous dermatitis, for example, has an incidence ranging from 2.2% to 23.5% in patients undergoing anti-TNF alpha therapy.12 Depending on severity, gentle skin care, liberal emollients, topical steroids, biopsy, or referral to dermatology is recommended.
Autoimmunity. Patients on TNF alpha inhibitors can develop autoimmune conditions that include alopecia (both autoimmune and scarring), dermatomyositis, sarcoidosis, and antiphospholipid syndrome. Specifically, leukocytoclastic vasculitis and TNF alpha inhibitor-induced lupuslike syndrome (TAILS) will be discussed below.
Leukocytoclastic vasculitis associated with TNF alpha inhibitors typically manifests as a cutaneous small-vessel vasculitis. Discontinuation of therapy is required in 94% to 100% of patients with TNF alpha inhibitor-induced leukocytoclastic vasculitis, and initiation of systemic prednisone or other immunosuppressive medication or both is sometimes required or recommended.13
TAILS, a form of drug-induced lupus, is rare (incidence < 1%), most commonly affects middle-aged women, and presents weeks to years after starting the TNF alpha inhibitor, particularly infliximab and etanercept.13 A maculopapular rash, malar rash, photosensitivity, and alopecia are common skin manifestations, seen in 72% of patients. Noncutaneous manifestations include arthritis, serositis, myositis, anemia, leukopenia, renal, and neurologic disorders.
If TAILS is suspected, patients can be screened for laboratory findings seen in lupus. Positive results for antibodies occur as follows: antinuclear antibody 91%, anti-dsDNA 64%, and antiphospholipid antibody 11% to 50%.13 Antihistone is also frequently found. In a study of 53 patients with rheumatoid arthritis receiving infliximab, the prevalence of antinuclear antibody at a dilution greater than 1:100 increased from 24% at baseline to 77% at 30 weeks and 69% at 54 weeks.13 Other studies in patients with rheumatoid arthritis have shown induction of antinuclear antibody and anti-dsDNA after the use of infliximab and etanercept.13
Of note, in some conditions such as rheumatoid arthritis, patients may already have underlying lupus features. However, TNF alpha inhibitors may trigger additional lupus features, leading to a diagnosis of TAILS. Withdrawal of the TNF alpha inhibitor and referral to dermatology or rheumatology are recommended in these cases.
Treatment for TAILS generally includes topical steroids, antimalarials, and possibly switching to another TNF alpha inhibitor.
Malignancy risk
Findings are mixed on whether TNF alpha inhibitors increase the risk of nonmelanoma skin cancer.14 In a meta-analysis of 4 observational studies with 28,000 patients, the risk of non-melanoma skin cancer was significantly higher among patients exposed to these drugs.14 However, the data are confounded by past or concomitant use of phototherapy or other immunosuppressive agents.
There is some evidence to suggest that patients receiving methotrexate, commonly used in rheumatoid arthritis, are at increased risk of nonmelanoma skin cancer, possibly due to the photosensitizing nature of methotrexate.15 One study in particular15 examined the rate of development of a second nonmelanoma skin cancer in 9,460 patients with rheumatoid arthritis or inflammatory bowel disease. It found that anti-TNF use may increase the nonmelanoma skin cancer risk when used in combination with methotrexate. However, further study is needed to eliminate confounding factors.
The link between melanoma and TNF alpha inhibitors is also not straightforward. In a Swedish cohort study,16 there was a higher risk of a first invasive melanoma in patients with rheumatoid arthritis receiving TNF alpha inhibitors than in those not treated with them. Another study,16 however, examined 130,315 patients who had rheumatoid arthritis and found 287 first-time melanomas. The risk was slightly higher than in the general population in the entire cohort and in those on TNF alpha inhibitors, but the differences were not statistically significant, and the overall absolute incidence was quite low.
Given the mixed findings, it is therefore reasonable that all patients treated with a TNF alpha inhibitor undergo skin cancer surveillance for both melanoma and nonmelanoma skin cancer, use broad-spectrum sunscreens, and practice sun avoidance and skin self-examination. If malignant melanoma is found, it is reasonable to stop the TNF inhibitor.
EPIDERMAL GROWTH FACTOR RECEPTOR INHIBITORS
EGFR is a transmembrane glycoprotein that, when activated, leads to the autophosphorylation of tyrosine kinase receptor, initiating a cascade of downstream signaling pathways involved in regulating cellular proliferation, differentiation, and survival. EGFR inhibitors block this pathway in tumor cells and are predominantly used in non-small cell lung cancer, colorectal cancer, pancreatic cancer, and head and neck cancer. Examples include gefitinib, cetuximab, erlotinib, and panitumumab (Table 2).
Cutaneous adverse effects of epidermal growth factor receptor inhibitors
Acneiform reactions
The most common cutaneous reaction with EGFR inhibitors is a widespread papulopustular acneiform eruption consisting of erythematous follicle-based papules and pustules without comedones.
More than half of patients taking these drugs experience an acneiform eruption. It is usually mild or moderate but can be severe in a minority of cases. The acneiform eruption is often dose-dependent and begins within 1 week of treatment.17
The lesions commonly present on the face and trunk, spare the palms and soles, and are associated with pruritus.
While management depends on the severity, consultation with a dermatologist is recommended for most patients, particularly if the reaction lasts more than 2 weeks or is severe. Prevention can include medications such as minocycline or doxycycline. Treatment can include topical and systemic corticosteroids, antibiotics, and oral isotretinoin. If there is pruritus, oral histamine 1 (H1) antihistamines can be used. Gamma aminobutyric acid agonists such as gabapentin and pregabalin can be used as second-line treatments for itching.
Toenail inflammation
A study of 10 patients suggested paronychial inflammation, commonly with pyogenic granuloma-like lesions, as another cutaneous manifestation.18 Paronychia in the great toe often occurs first, and secondary bacterial infection (commonly Staphylococcus aureus) can occur as well.
Treatment with topical antibiotics, topical corticosteroids, electrodessication for larger lesions, and, more rarely, photodynamic therapy, can be effective.
Other reactions
EGFR inhibitors have also been associated with hair changes including development of brittle, fine, and curly hair on the scalp and extremities.
Xerosis with desquamation, small aphthous ulcerations of the oral and nasal mucosa, photosensitivity, and urticaria have also been noted.
Cases of Stevens-Johnson syndrome-toxic epidermal necrolysis have been associated with erlotinib therapy, but the incidence is low.19 Discontinuation is recommended if any sign of a bullous or exfoliative eruption occurs.
SMALL-MOLECULE TYROSINE KINASE INHIBITORS
TKIs block intracellular signaling pathways that regulate cellular functions such as proliferation and differentiation in tumor cells. Different small molecules may target different components of the tyrosine kinase signaling cascade. Examples include imatinib, dasatinib, nilotinib, ponatinib, bosutinib, sorafenib, sunitinib, pazopanib, axitinib, vandetanib, dovitinib, vemurafenib, dabrafenib, and ruxolitinib (Table 3).
Cutaneous adverse effects of small-molecule tyrosine kinase inhibitors
Imatinib
Imatinib is commonly used to treat Philadelphia-chromosome-positive chronic myelogenous leukemia (Ph+CML) and gastrointestinal stromal tumors. It can trigger skin eruptions, sometimes in up to 1 in 5 (20%) treated patients. A study of 532 patients with chronic-phase CML treated with imatinib daily found that 32% reported a rash or related cutaneous event.20 Most commonly, the rash presented as an exanthematous papular eruption.
When mild, this rash will resolve spontaneously. However, more severe skin eruptions may require stopping treatment for 2 weeks, and then restarting at a lower dose. Upon reintroduction, a potential strategy is to temporarily add an oral corticosteroid to minimize risk of a repeat cutaneous reaction.
Beyond rash, one prospective study of 54 patients on imatinib found that 7% developed photosensitivity and 7% developed a psoriasiform eruption.21 Imatinib has also been linked to Stevens-Johnson syndrome, acute generalized exanthematous pustulosis, and Sweet syndrome (acute febrile neutrophilic dermatosis). Discontinuation is recommended in these cases. For the latter two, the decision to attempt retreatment depends on the extent of the reaction and if there are therapeutic alternatives.
Second-generation TKIs
Dasatinib, nilotinib, ponatinib, and bosutinib are second-generation TKIs that are used for treatment of Ph+CML. There are a number of cutaneous findings to be aware of when encountering these drugs.
Phase 1 and 2 studies of dasatinib found that of 911 patients, 35% had cutaneous eruptions, including localized and generalized erythema, papular eruptions, and pruritus.22
In phase 1 and 2 studies of nilotinib, 20% to 28% suffered a nonspecific rash, 15% to 24% had pruritus, and 12% had dry skin.23
Bosutinib can cause adverse dermatologic concerns in 20% to 44% of patients, including erythema, maculopapular eruption, pruritic rash, allergic dermatitis, acne, folliculitis, and skin exfoliation.24
Treatments for the above reactions generally include topical and systemic corticosteroids, isotretinoin, and oral H1 antihistamines, depending on the specific concern.
Sorafenib and sunitinib are multitargeted TKIs whose most common cutaneous effects involve hand-foot skin reactions. A metaanalysis involving 6,011 patients on sorafenib found hand-foot skin reactions occurred in 39%, while less common cutaneous reactions included all-grade rash or desquamation (35.4%), alopecia (25.5%), pruritus (14%), and dry skin (14.1%).25 Patients treated with sunitinib or sorafenib who develop handfoot skin reactions tend to develop localized tender lesions in friction areas that can become blistered or hyperkeratotic. Handfoot skin reactions appear more commonly with sorafenib than with sunitinib, and their severity with higher doses is a pattern found specifically in sorafenib recipients.26 For mild hand-foot skin reactions, dosing of the medication can remain the same, and topical emollients, topical urea, or salicylic acid may be effective. In more severe cases, treatment may require a topical corticosteroid or temporary reduction in dose.
Sorafenib has also been associated with cutaneous squamoproliferative lesions such as keratoacanthomas and squamous cell carcinomas.27 Of note, lesions have the potential to regress upon cessation of therapy. Complete surgical excision, similar to treatment of those not on the drug, can be employed in these cases.
Pazopanib, axitinib, vandetanib, and dovitinib are all multitargeted TKIs. Pazopanib is used for advanced renal cell carcinoma and soft-tissue sarcoma. When studied in 290 patients with renal cell carcinoma, changes in hair color occurred in 38% of recipients.28
Axitinib is approved for the treatment of advanced renal cell carcinoma. Of 984 patients, 29.2% had hand-foot skin reactions.29 Vandetanib is used for patients with medullary thyroid cancer and lung cancer. It can present with skin reactions of dermatitis, acneiform eruption, dry skin, pruritus, photosensitivity, or hand-foot skin reactions in 28% to 71% of 30 patients.30
Dovitinib is used in renal cell carcinoma and melanoma. It has been reported to cause acneiform eruptions and eruptive facial milia and comedones (Figure 3).31 Topical antiseptics, topical antibiotics, oral antibiotics, and systemic isotretinoin can be used for treatment. A short course of a low-dose systemic corticosteroid can also be useful to control inflammation.31
Acneiform eruption in a patient receiving dovitinib for glioblastoma.
Vemurafenib and dabrafenib are inhibitors of the kinase domain in mutant BRAF (a serine-threonine kinase) and are used for treatment of metastatic melanoma with a V600E BRAF mutation. In clinical trials in 675 patients, vemurafenib was associated with a rash in 18% of patients, photosensitivity in 12%, squamous cell carcinoma or keratoacanthoma in 18% to 26%, and alopecia in 8%.32 Dabrafenib also has been associated with development of keratoacanthomas or well-differentiated cutaneous squamous cell carcinoma in 6% to 26% of patients.33 Treatment of these skin lesions can include phototherapy, intralesional methotrexate, retinoids, or surgical excision.
Ruxolitinib is a Janus-associated kinase inhibitor used in the treatment of myelofibrosis and polycythemia vera. Ruxolitinib is particularly associated with the development of skin cancer, as 17.1% of patients on the therapy developed basal cell carcinoma or squamous cell carcinoma compared with 2.7% of patients on alternate available therapy for myelofibrosis (Figure 4).34 A case series reported 5 patients with a history of myelofibrosis treated with ruxolitinib who developed multiple skin cancers with aggressive features, including a lentigo maligna melanoma.34
Eruptive squamous cell carcinoma keratoacanthomas in a patient receiving ruxolitinib for primary myelofibrosis.
CELL SURFACE-TARGETED MONOCLONAL ANTIBODIES
Monoclonal antibodies are drugs directed against specific antigens on cells that cause disease. These drugs may assist in immune modulation, cell killing, or blocking a physiologic ligand-receptor interaction. Not surprisingly, monoclonal antibodies are used in the treatment of immunologic diseases and cancer therapy. Although the number of monoclonal antibodies designed as drugs has been increasing substantially since 1985, common ones include rituximab, anakinra, tocilizumab, ipilimumab, nivolumab, pembrolizumab, avelumab, and tofacitinib (Table 4).
Cutaneous adverse effects of cell surface-targeted monoclonal antibodies
Rituximab, an anti-CD20 monoclonal antibody
Rituximab is a chimeric murine-human monoclonal antibody against CD20 used in rheumatoid arthritis, autoimmune disorders, and lymphoproliferative disorders. While dermatologically it is relatively benign, it has been reported to cause infusion reactions. Standard practice is to premedicate with acetaminophen and diphenhydramine 30 minutes before the first and second infusions.
Serum sickness has also been reported with rituximab, and is seen visibly as a morbilliform skin eruption with acral accentuation.35 Treatment for this reaction includes pulse methylprednisolone therapy, which can be effective in resolving symptoms over 48 hours.
Less commonly, Stevens-Johnson syndrome-toxic epidermal necrolysis and vesiculobullous dermatitis can occur with rituximab, in which case discontinuation is recommended.
Other monoclonal antibodies
Other commonly used monoclonal antibodies include anakinra, tocilizumab, and ipilimumab.
Anakinra is a recombinant human interleukin 1 receptor antagonist used to treat rheumatoid arthritis, systemic juvenile idiopathic arthritis, adult-onset Still disease, and, in select patients, recurrent pericarditis. Case reports note new-onset psoriasis with this drug, as well as injection-site reactions.36
Tocilizumab is an anti-human interleukin 6 receptor antibody used for rheumatoid arthritis and giant cell arteritis. It rarely presents with skin rash, but is most notable for hypersensitivity reactions upon infusion.37
Ipilimumab is a monoclonal antibody directed against cytotoxic T-lymphocyte antigen 4 used to treat patients with advanced melanoma.
In 41 patients treated with ipilimumab, 34.1% developed cutaneous adverse events that included rash (7.3%), folliculitis (7.3%), mucositis (2.4%), rosacea (2.4%), eczema (2.4%), acneiform eruption (2.4%), syringometaplasia mucinosa (2.4%;), Stevens-Johnson syndrome (2.4%), and vitiligo (4.9%). Approximately 5% of the patients complained of severe xerosis and 10% of pruritus.38
Treatment for these cutaneous manifestations is similar to that described in previous sections.
Nivolumab, pembrolizumab, and avelumab bind to programmed cell death ligand-1 (PD-L1), enhancing the host immune response by preventing tumor cells from suppressing endogenous T-cell activity. Cutaneous eruptions described with these medications include lichenoid, bullous, psoriasiform, macular, and morbilliform morphologies. Cases of Stevens-Johnson syndrome-toxic epidermal necrolysis have also been reported (Figure 5).
Toxic epidermal necrolysis in a patient receiving pembrolizumab for Sézary syndrome.
Treatment with topical corticosteroids, systemic steroids, or discontinuation of the anti-PD-L1 inhibitor may be effective depending on the severity of the eruption.39
BE ON THE LOOKOUT
Biologic medications are becoming critical in medicine, for treating conditions ranging from autoimmune diseases to metastatic cancers. They are reducing mortality and substantially improving quality of life.
It is therefore important that physicians be armed with knowledge about the cutaneous adverse events of these medications and basic treatment steps. For example, knowing when to reduce the dose or discontinue the drug, supplement with topical or oral steroids or antibiotics, or refer to a dermatologist will be highly useful when caring for patients on these biologics. These innovative medications will only reach their maximum effectiveness when we as providers understand and manage adverse events appropriately.
Acknowledgment
The authors would like to thank Mrs. Janine Sot, MBA, for her expertise in creating the figures in this manuscript.
Footnotes
Dr. Fernandez has disclosed financial relationships (consulting, membership on advisory committee or review panels, research, independent contracting, or teaching and speaking) with commercial interests (Abbview Pharmaceuticals, Corbus Pharmaceuticals, Mallinckrodt, Novartis, Pfizer, and UCB).
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.