A 40-year-old woman presented with altered mental status, having been found by her family after she had been in the bathroom for more than 7 hours. She was on chemotherapy with mFOLFOX6 for metastatic rectal cancer. Her regimen consisted of 5-fluorouracil (5-FU) in a 400-mg/m2 bolus and 2,400 mg/m2 by continuous infusion for 3 days, levofolinate 200 mg/m2, and oxaliplatin 85 mg/m2. She was currently on her 10th course and had been experiencing general malaise and appetite loss. At the onset of her current symptoms, her cumulative dose of 5-FU was 40.2 g. She had no known metastasis to the liver, nor did she have a history of liver disease.
Her Glasgow Coma Scale score on arrival was 10 on a scale of 15, with the following elements:
Eye opening 3 of 4 (opens eyes to sound)
Verbal response 1 of 5 (no response)
Motor response 6 of 6 (obeys commands).
Her eyes were deviated upward. She had urinary incontinence.
On arterial blood gas analysis, oxygen and carbon dioxide levels were normal; lactate was elevated (4.1 mmol/L) without acidemia (pH 7.445, bicarbonate 24.8 mmol/L).
Results of a complete metabolic panel revealed elevated serum ammonia (109 μg/dL) and blood urea nitrogen (23 mg/dL) levels, and normal levels of electrolytes, glucose, hepatobiliary markers, serum creatinine, and serum thiamine (27 ng/mL). A urine drug screening test was negative.
Electroencephalography showed diffuse slow and triphasic waves without epileptiform patterns.
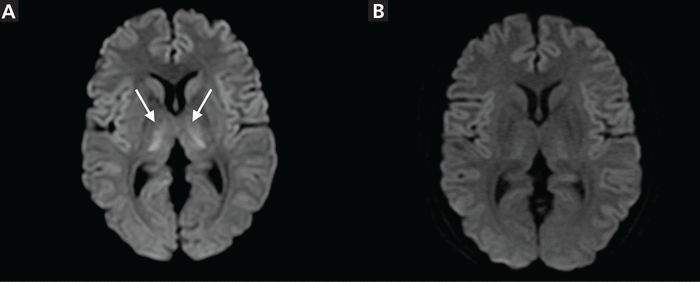
Diffusion-weighted brain magnetic resonance imaging (MRI) showed symmetrical, bilateral high-intensity areas in the insular cortex, cingulate gyrus, and thalamus (Figure 1a).
(A) Symmetrical, bilateral, high-intensity areas in the insular cortex, cingulate gyrus, and thalamus (arrows) on diffusion-weighted brain magnetic resonance imaging. (B) The high-intensity area in the thalamus disappeared and those in the insular cortex and cingulate gyrus decreased on hospital day 2.
5-FU was discontinued. Her mental status recovered, and MRI findings normalized by hospital day 2 (Figure 1b). Her symptoms were diagnosed as 5-FU–induced encephalopathy, and she was discharged on day 5. Her chemotherapy regimen was changed, and no mental status changes recurred.
5-FU–INDUCED ENCEPHALOPATHY
5-FU is one of the most widely used anticancer drugs. It can induce encephalopathy that presents with altered mental status or seizures, although this effect is rare, with an incidence of 0.6%.1 The encephalopathy can present as hyperammonemic encephalopathy, leukoencephalopathy, or Wernicke encephalopathy. Risk factors include azotemia, dehydration, and bacterial infection.2
Main mechanisms
Krebs cycle suppression, caused by fluoroacetate, a 5-FU catabolite, inhibits the adenosine triphosphate-dependent urea cycle, leading to hyperammonemia.3 This mechanism also produces lactic acid, causing hyperlactatemia.
Dihydropyrimidine dehydrogenase (DPD) deficiency. DPD is an enzyme involved in 5-FU catabolism. DPD deficiency leads to 5-FU accumulation, with neurotoxic effects such as demyelination.4 5-FU also increases cellular thiamine metabolism, thereby causing Wernicke encephalopathy.5
Although the DPD level was not measured in this case, the mechanism likely involved Krebs cycle suppression rather than DPD deficiency because serum ammonium and lactate levels were elevated.
Diagnosis and prognosis
The diagnostic criteria for 5-FU–induced encephalopathy include development of encephalopathy during or shortly after 5-FU administration, along with exclusion of other metabolic, somatic, and drug-related causes.6 The differential diagnosis includes stroke, nonconvulsive status epilepticus, other encephalopathy (eg, uremic, hepatic, drug-induced), infection, and psychogenic disorders. However, the history of recent 5-FU administration is crucial.
This disorder has a diverse MRI presentation. In the leukoencephalopathy type, the lesions are found in the deep white matter and corpus callosum.1 The gray matter, including the bilateral basal ganglia, thalamus, and para-sagittal frontal cortices can occasionally be involved, as in our patient.7 Regardless of the presentation, abnormal MRI findings improve after 5-FU is stopped.1,7 Bilateral, symmetrical lesions in the insular cortex and cingulate gyrus, as in our patient, are characteristics of hyperammonemic encephalopathy.8
Discontinuing 5-FU and providing supportive therapy usually lead to rapid symptom resolution,8 although fatal outcomes have been reported.9 Uridine triacetate, the antidote for 5-FU, has been proposed as a treatment for severe 5-FU toxicity and should be considered for severe cases.10
Acknowledgment
The authors would like to sincerely thank Ms. Ryoko Yoshida for editing the figure.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.