ABSTRACT
Although echocardiography is fundamental in diagnosing infective endocarditis, sometimes it reveals no evidence of endocarditis while clinical indicators remain consistent with the diagnosis. In such cases, repeat imaging is necessary, and the appropriate timeline for it and whether it should be done with transthoracic echocardiography (TTE), trans esophageal echocardiography (TEE), or an advanced imaging method are important.
Guidelines recommend TTE as the first test for suspected infective endocarditis, usually combined with TEE.
If imaging findings are negative in a patient in whom the disease is strongly suspected, imaging should be repeated 3 to 7 days later.
TEE is more sensitive than TTE (which is quicker and noninvasive) for diagnosing and characterizing infective endocarditis, but even using TEE, results may be falsely negative before vegetations or other findings of endocarditis are detectable.
Multidetector cardiac computed tomography may be used to better visualize prosthetic valve vegetations, abscesses, pseudoaneurysms, and dehiscence.
18F-fluorodeoxyglucose positron emission tomography/computed tomography may be indicated for patients with prosthetic valves or cardiac implantable electronic devices.
A 68-year-old woman with a prosthetic aortic valve presents with fever and acute right lower limb pain. Blood cultures grew Staphylococcus aureus. Transthoracic echocardiography (TTE) demonstrates satisfactory valve function with no obvious vegetations. Due to ongoing concern about infective endocarditis, transesophageal echocardiography (TEE) is performed. Again, no obvious prosthetic aortic valve vegetations are found.
Infective endocarditis can be a challenge to diagnose. Echocardiography is the cornerstone of evaluation, but what should be done if no echocardiographic evidence of infective endocarditis is found in a patient who continues to have bacteremia? Key questions in such a situation include:
When should echocardiography be repeated?
Should it be TTE or TEE, or should a more advanced imaging method be used?
This article reviews the use of echocardiography for diagnosing infective endocarditis, the emerging roles of advanced imaging methods, current guidelines and their limitations, and special considerations for patients at high risk.
AN OLD PROBLEM IN A NEW DEMOGRAPHIC
Despite advances in imaging and diagnosis, infective endocarditis, an infection of the endocardium or heart valves, remains a serious disease with high morbidity and mortality rates.1,2 It is most often precipitated by bacteremia; bacteria (most commonly S aureus or viridans streptococci) enter the bloodstream and adhere to damaged or abnormal endothelium, resulting in colonization and proliferation with monocyte recruitment, thrombosis, and inflammation.3
Infective endocarditis has undergone a demographic shift in recent decades. Formerly it was most often seen in patients with rheumatic or congenital heart disease, but now it is likelier to be associated with hemodialysis, immunosuppression, prosthetic valves, other cardiac devices, or intravenous drug use.4
ECHOCARDIOGRAPHY IS ESSENTIAL
Infective endocarditis is diagnosed by the modified Duke criteria, with major and minor criteria used to determine whether it is definitely or probably present.3 Major criteria include positive blood cultures of typical microorganisms consistent with the disease and echocardiographic evidence.5 Echocardiography is the key imaging method for diagnosing infective endocarditis and assessing its prognosis.6
The major echocardiographic criteria for diagnosing infective endocarditis are vegetations (ie, oscillating or nonoscillating intracardiac masses on a valve, other endocardial structure, or implanted intracardiac material), an abscess, and new dehiscence of a prosthetic valve. Valve destruction, aneurysm, or perforation suggest the diagnosis.7
TTE is typically performed initially. It is rapid, noninvasive, widely available, and highly specific, justifying its use as a first-line screening tool.6,8 However, suboptimal findings are especially likely in patients with prosthetic valves because of poor resolution of prosthetic leaflets due to acoustic shadowing.9 TEE is used when TTE imaging is suboptimal, or when clinical suspicion remains high in a patient with persistent bacteremia despite negative findings on TTE.
Infective endocarditis is a dynamic process, and infective valvulitis may be present before a discrete vegetation is visible using TTE or TEE.2 Patients without echocardiographic findings but who still are suspected clinically of having infective endocarditis may either be in the early stages of the disease or have a different disease process.
USE BOTH TTE AND TEE FOR MANY PATIENTS
Guidelines from the European Society of Cardiology (ESC),10 American Heart Association (AHA),4,11 and American College of Cardiology (ACC)11 are summarized in Table 1.
ESC guidelines
The ESC guidelines10 say to perform TTE as soon as infective endocarditis is suspected.10 TEE should also be performed in many cases because of its superior image quality, spatial resolution, and sensitivity.7 This applies to a variety of clinical scenarios, including when:
TTE is negative, but a high clinical suspicion of infective endocarditis remains
TTE findings are of poor quality
TTE demonstrates abnormal changes, but the valvular structure needs to be further delineated and involvement of other valvular structures needs to be ruled out
The patient has a prosthetic valve or intracardiac device.
The only scenario in which TEE is not usually performed after TTE yields negative results is in patients who have bacteremia but a low clinical suspicion of infective endocarditis.10 This includes those with bacteremia as a result of line-related infections whose symptoms resolve after the line is removed, and those without high-risk features (eg, a permanent intracardiac device, dialysis dependency, or bacteremia for at least 4 days).12,13
AHA and ACC guidelines
Recommendations from the AHA4 and AHA/ACC11 are similar to those of the ESC.10 In patients suspected of having infective endocarditis on the basis of the modified Duke criteria (including 2 positive blood cultures), TTE is recommended to characterize anatomic features.11 TEE is recommended if TTE is not diagnostic, intracardiac leads are present, or complications are suspected. TTE should be performed in all cases of suspected native valve infective endocarditis, with follow-up TEE in 3 to 5 days if clinical findings change or suspicion remains high despite negative findings on TTE. Intraoperative TEE is recommended for patients undergoing surgery.
Adjuvant imaging with multidetector computed tomography (MDCT) can be considered in patients who have unremarkable findings on TTE and TEE if prosthetic or paravalvular infections continue to be suspected.11
WHEN SHOULD TTE OR TEE BE REPEATED IF NEGATIVE, BUT BACTEREMIA PERSISTS?
There is some controversy as to when to repeat echocardiography in cases in which both TTE and TEE are unremarkable but the clinical suspicion of infective endocarditis is high. Evidence gaps exist for optimal timing of repeat echocardiography according to patient pathology, risk status, and outcomes.
In these situations, a repeat TEE should be scheduled (ESC guidelines: 7–10 days after an initial negative TEE; AHA guidlines: 3–5 days after an initial TEE). But it is especially important that this should be done only if there is ongoing clinical suspicion for infective endocarditis.
Some studies suggest repeating echocardiography 7 to 10 days later, while others recommend 5 to 7 days (or even earlier in S aureus infection).7,14 Thus, the ESC guidelines recommend repeating TTE or TEE, or both, 7 to 10 days later in cases of an initially negative examination if clinical suspicion of infective endocarditis remains high.10
Sochowski and Chan8 studied 105 patients who underwent TEE for suspected infective endocarditis, of whom 65 had a negative study. In 56 of these 65 patients, an alternative diagnosis was made, in another 5, infective endocarditis was diagnosed by repeat TEE, and the other 4 patients were treated for infective endocarditis without a definitive diagnosis. Gram-positive bacteremia and prosthetic valves were more common in the group with proven infective endocarditis than in those with suspected infective endocarditis but negative findings on TEE, although the difference was not statistically significant (P = .07), possibly due to the small sample size. The study reported that the optimal timing for repeat imaging varied by patient.
Other indications for repeating echocardiography include the new onset of complications of infective endocarditis (eg, a new murmur, embolism, heart failure, abscess, atrioventricular block), persisting fever, and follow-up of suspected, uncomplicated infective endocarditis.4
TTE or TEE for follow-up?
Evidence is lacking on whether TTE or TEE is more appropriate when echocardiography should be repeated.10 TEE remains superior in assessing for evidence of infective endocarditis. Studies from the 1980s and 1990s found TEE to be more sensitive than TTE in detecting valvular vegetations: 100% vs 63% (N = 96),15 94% vs 44% (N = 66),16 and 87% vs 69% (N = 64).17 Daniel et al18 also found TEE to be more sensitive than TTE for detecting valvular abscesses: 87% vs 28% (n = 118). However, these studies were small and were done decades ago, and echocardiography has undergone many advances since then, including 3-dimensional TEE.
NATIVE VS PROSTHETIC VALVE
TEE is more sensitive than TTE for diagnosing infective endocarditis regardless of whether a native or prosthetic valve is involved. However, some differences should be kept in mind.
Use TEE for native valve evaluation
In native valve endocarditis, the diagnostic accuracy of TTE depends on the size of vegetations and underlying valvular disease, with sensitivity ranging from 40% to 63% compared with 90% to 100% for TEE.19
Reynolds et al20 evaluated TTE incorporating harmonic imaging for 51 vegetations seen on TEE. The sensitivity of TTE in detecting native valve vegetations was only 55%, and the size of the vegetation affected the sensitivity. When TTE was positive, vegetation size on TEE was significantly larger than when TTE was falsely negative, which was true for aortic valves (11.2 ± 3.4 mm vs 5.8 ± 3.6 mm, P = .001) and for mitral valves (12.9 ± 4.1 mm vs 7.9 mm ± 5.0 mm, P = .01). Furthermore, TEE was able to reveal additional diagnoses not seen by TTE in 7 patients (14%), including aortic valve prolapse, aneurysm, and vegetations on intervalvular fibrosa and pacemaker wires.
Use TEE and consider advanced imaging for prosthetic valves
In prosthetic valve endocarditis, vegetations are more difficult to detect, and TEE is typically used in conjunction with TTE for diagnosis.19
Use of MDCT
In a systematic review and meta-analysis of 20 studies in 496 patients, Habets et al21 found the pooled sensitivities for detecting prosthetic valve vegetations were 82% using TEE, 88% using TEE plus MDCT, and 29% using TTE alone. The pooled sensitivities for detecting life-threatening periannular complications (eg, abscesses and mycotic aneurysms) were 86% using TEE, 100% using TEE and MDCT, and 36% using TTE alone.
Use of FDG-PET/CT
The added diagnostic value of advanced cardiac imaging is reflected in the 2015 ESC and AHA/ACC guidelines, which recommend combined 18F-fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) for diagnosing prosthetic valve endocarditis but not for native valve endocarditis.4,10
In 2019, San et al22 confirmed the rationale for this recommendation after evaluating FDG-PET/CT in 64 patients with native valve endocarditis and 109 patients with prosthetic valve endocarditis. FDG-PET/CT was found not only to be a better diagnostic tool for prosthetic valve endocarditis than for native valve endocarditis (sensitivity 83% vs 16%), but it was also better for predicting major cardiac events, infective endocarditis recurrence, and new embolic events (P = .04).
CARDIAC DEVICE-RELATED INFECTIONS
Diagnosing infective endocarditis is challenging in patients who have an implantable cardiac device, and TEE is superior to TTE.
In 1994, Vilacosta et al23 used echocardiography to evaluate 10 patients with permanent transvenous pacemakers who were suspected of having infective endocarditis. TTE was positive for pacemaker lead vegetations in 2 of these patients, while TEE was positive in 7 patients.
In 2013, Narducci et al24 conducted a prospective observational study in 162 patients comparing TEE with intracardiac echocardiography in diagnosing cardiac device-related infection. Intracardiac echocardiography had high diagnostic accuracy for detecting intracardiac masses (sensitivity 100%, specificity 82.8%, positive predictive value 65.6%, and negative predictive value 100%, P < .001). However, because this method is invasive and needs to be performed in the cardiac catheterization laboratory, it may not be appropriate for other types of infective endocarditis, for which TTE or TEE have sufficient diagnostic capacity.
PET/CT has also been explored for diagnosing infective endocarditis in patients with implantable cardiac devices.25
CAUSATIVE ORGANISMS
A variety of pathogens have been implicated in causing infective endocarditis. A prospective cohort study of 2,781 patients with infective endocarditis in 58 hospitals in 25 countries found the 5 most common pathogens to be:
S aureus (31%)
Viridans streptococci (17%)
Enterococci (11%)
Coagulase-negative staphylococci (11%)
Streptococcus bovis (7%).
Rarer causative organisms include other streptococci, fungi, and HACEK organisms (Haemophilus aphrophilus, Aggregatibacter [previously Actinobacillus] actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae).2
S aureus bacteremia
Infective endocarditis worsens the prognosis of S aureus bacteremia, and patients with an intracardiac device are at especially high risk of having infective endocarditis in the setting of S aureus bacteremia.26
Initial evaluation with TEE has been suggested for patients with S aureus bacteremia because of the high complication rate associated with failure of diagnosis in this setting, and the higher sensitivity and specificity of TEE than TTE alone.27 However, the ESC guidelines recommend TTE as the first-line imaging in S aureus bacteremia, and a repeat investigation with TTE, TEE, or both within 7 to 10 days.7,10 A 2014 study by Barton et al27 found that more than half of patients (132 of 256) with S aureus bacteremia had an initially negative TTE, of which only 6 were subsequently diagnosed with infective endocarditis by TEE (negative predictive value 95% for TTE in S aureus bacteremia), suggesting that TTE may be satisfactory in follow-up of initially TTE-negative patients with uncomplicated S aureus bacteremia.
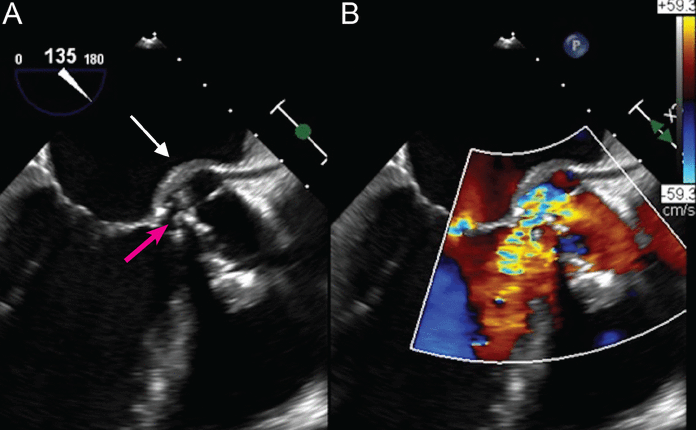
Figure 1 shows an aortic root abscess, a possible complication of S aureus bacteremia-associated infective endocarditis.
(A) Transesophageal echocardiography, mid-esophageal long-axis view, demonstrates a prominent aortic root abscess cavity (white arrow) posteriorly in a patient with a prosthetic aortic valve. Also note partial dehiscence of the aortic bioprosthesis (red arrow). (B) Color Doppler analysis demonstrates significant aortic regurgitation between the aortic bioprosthesis and the left ventricular outflow tract through the prominent abscess cavity.
Enterococcal species
An estimated 3% to 10% of patients with enterococcal bloodstream infections develop infective endocarditis.27 Unlike S aureus bacteremia, for which the 2015 AHA guidelines advise initially performing TEE, this is generally not recommended for enterococcal bacteremia.4
Bouza et al28 proposed the NOVA score to identify those patients with enterococcal bacteremia with high enough risk of infective endocarditis to warrant TEE. The NOVA score consists of the following:
Persistent bacteremia (defined as 3 of 3 positive blood cultures or the majority positive if more than 3) = 5 points
Unknown source of bacteremia = 4 points
History of valve disease = 2 points
Heart murmur auscultated = 1 point.
The authors concluded that a score of 4 points or more warrants TEE, with a sensitivity of 100% and specificity of 29% for detecting infective endocarditis.
In a retrospective cohort study, Dahl et al29 evaluated a modified NOVA score (2 of 2 positive blood cultures earning 5 points, and all other criteria unchanged). Seventy-six of 78 patients with enterococcal infective endocarditis had a NOVA score of at least 4, translating into a sensitivity of 97% and a negative predictive value of 95%. The findings support the use of the NOVA score in identifying patients with a low risk of infective endocarditis for whom investigation with TTE may be sufficient. Timing for a repeat echocardiogram, however, is recommended at 7 to 10 days regardless, according to ESC guidelines for S aureus and Enterococcus faecalis bacteremias.7
ALTERNATIVE IMAGING METHODS
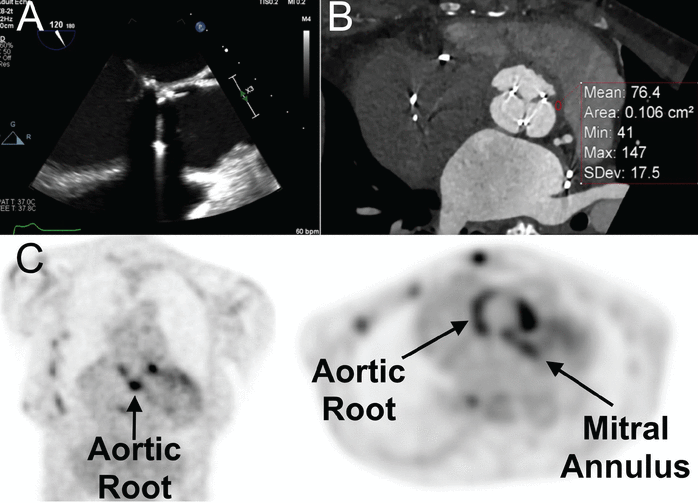
With advances in cardiovascular imaging, evaluating infective endocarditis is no longer limited to conventional echocardiography and may also include other methods, eg, MDCT, FDG-PET/CT, and other functional imaging (Figure 2).
Utility of adjuvant advanced cardiovascular imaging—multidetector cardiac computed tomography (MDCT) and 18F-fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT)—in the diagnosis of infective endocarditis. A 68-year-old woman with Staphylococcus aureus bacteremia in the setting of a bioprosthetic aortic valve developed fever and acute right lower limb pain. Initial transesophageal echocardiography (mid-esophageal long-axis view) showed no obvious vegetation associated with the bioprosthesis (A). Due to ongoing clinical suspicion for prosthetic aortic valve endocarditis, MDCT was performed (B) and, at the level of the aortic root, showed abnormal thickening with elevated Hounsfield units (mean: 76.4 units), highly suspicious for periprosthetic aortic root abscess. FDG-PET/CT (C), demonstrated abnormal increased activity at the aortic root and mitral annulus (arrows).
Echocardiography is limited in that early vegetations are often difficult to detect on TTE and TEE if their size is below the resolution of the transducer. Also, small vegetations can be hard to differentiate from degenerative valvular thickening or calcification.8 The ESC guidelines provide some guidance for using alternative imaging methods, such as MDCT and FDG-PET/CT, to increase the sensitivity of the Duke Criteria, but evidence is limited.10 Despite the growing use of alternative imaging modalities such as MDCT and PET/CT, few studies exist comparing them with TTE and TEE for diagnosing infective endocarditis. As adjuvant imaging modalities become more widely used, more prospective trials are needed to increase the evidence base.
3-D TEE has advantages over 2-D TEE
A 2014 study by Berdejo et al30 compared 3-D TEE with conventional 2-D TEE in 60 patients with a definite diagnosis of infective endocarditis as demonstrated by vegetations seen on 2-D TEE. 2-D TEE underestimated the size of vegetations: the difference in maximum length between 3-D and 2-D TEE was 3.2 mm (95% CI 2.1–4.2 mm).
3-D TEE has potential advantages in evaluating paravalvular extension of infection, valve perforation, and prosthetic valve dehiscence.10 However, high-quality 3-D echocardiography relies on optimal 2-D imaging, from which the 3-D images are generated.
MDCT is better in some situations
Some studies have found MDCT to be better than echocardiography at identifying infective endocarditis. A review by Goddard et al31 found several studies showing MDCT to be equivalent or superior to echocardiography for identifying prosthetic vegetations, abscesses, pseudoaneurysms, and dehiscence.
A 37-patient study by Feuchtner et al32 found that MDCT detected valvular abnormalities in 28 of 29 patients with confirmed infective endocarditis, with a sensitivity of 97% and specificity of 88%. Moreover, MDCT detected paravalvular abscesses and pseudoaneurysms that were not detected by TEE in 3 patients.
MDCT may be superior in these scenarios, especially for evaluating paravalvular extension of infections. However, for typical findings, especially for detecting smaller, mobile vegetations, echocardiography with higher temporal resolution remains the preferred imaging method.31 In a study comparing MDCT with TEE in 75 patients with confirmed infective endocarditis, vegetations smaller than 10 mm were underdiagnosed by MDCT compared with TEE (detection rate 52.8% vs 94.4%).33 Moreover, MDCT assessment is dependent on the quality of the valve images and requires dedicated protocols. For instance, it may be difficult to interrogate the tricuspid valve precisely by MDCT.
FDG-PET/CT has an emerging role
Nuclear imaging techniques are becoming increasingly important for diagnosing infective endocarditis, especially for patients with equivocal TTEs or a negative TTE but a high clinical suspicion of infective endocarditis. FDG-PET/CT has been reported to reduce the rate of misdiagnosed infective endocarditis by detecting peripheral embolic and metastatic infectious events.34 Studies have shown its promising role as a diagnostic tool for infective endocarditis, particularly if related to a prosthetic valve or cardiac device; in such settings, FDG-PET/CT has been found to detect periprosthetic abscesses not identified by echocardiography.25,35,36
A 2020 meta-analysis of 1,358 patients found the pooled sensitivity of FDG-PET/CT to be 0.86 in infective endocarditis involving a prosthetic valve, 0.72 involving a cardiac device, and only 0.31 in native valve infective endocarditis.36
This imaging technique may also prove useful for monitoring clinical response to antimicrobial treatment.37
However, drawbacks include limited evidence of its cost-effectiveness and limited availability, only in tertiary centers with access to PET/CT scanners and appropriate imaging team support.37 In clinical practice, equivocal findings with mild uptake result in clinical ambiguity as to whether an infection is present. Due to limited resolution and valve mobility, the technique may not be sensitive in detecting vegetations smaller than 5 mm on native valves.38 In addition, other diseases associated with increased metabolic activity, including thrombi, atherosclerotic plaques, sarcoidosis, and primary and metastatic cardiac tumors, may cause false-positive findings, due to focal FDG uptake in the absence of infection.37
OUR ALGORITHM FOR EVALUATING SUSPECTED INFECTIVE ENDOCARDITIS
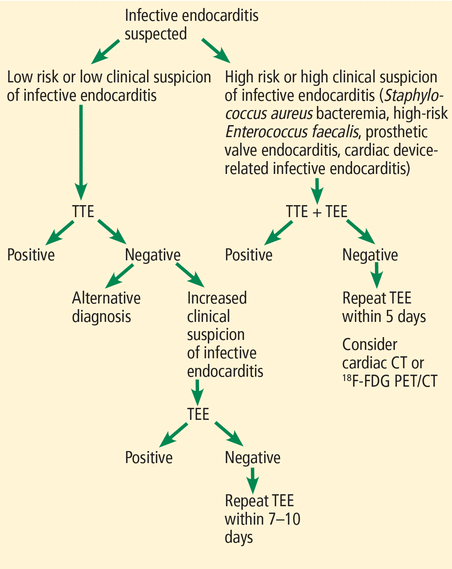
We propose a diagnostic pathway for evaluating suspected infective endocarditis based on the current literature and ESC and AHA/ACC guidelines (Figure 3). First, patients should be risk-stratified to guide the choice of initial imaging method. Per AHA/ACC guidelines, patients with persistent S aureus or E faecalis bacteremia, prosthetic valves, or cardiac devices should be characterized as high risk. They may require TEE imaging as a first-line investigation due its higher diagnostic accuracy. If initial TTE is negative and bacteremia persists in high-risk patients, then TEE should be performed within 5 days and consideration given to adjuvant imaging modalities. MDCT can be used to better visualize prosthetic vegetations, abscesses, pseudoaneurysms, and dehiscence. FDG-PET/CT may be used for patients with prosthetic valves or cardiac implantable electronic devices.
A proposed diagnostic algorithm for infective endocarditis.
TEE = transesophageal echocardiography; TTE = transthoracic echocardiography
If a low-risk patient has a negative TTE, the clinician should look for alternative diagnoses, ie, other than infective endocarditis. If clinical suspicion for infective endocarditis increases during the clinical evaluation, TEE should be conducted. If this yields a negative result, TEE should be repeated in 7 to 10 days.
CASE CONCLUDED
Due to ongoing clinical suspicion for prosthetic aortic valve infective endocarditis, despite apparently unremarkable echocardiographic imaging, adjuvant advanced imaging with dedicated cardiac CT and FDG-PET/CT were pursued (Figure 2). These studies helped confirm the diagnosis of prosthetic aortic valve infective endocarditis. The patient underwent re-do cardiac surgery successfully. At follow-up, the patient completed the course of antimicrobial therapy and was clinically well.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2021 The Cleveland Clinic Foundation. All Rights Reserved.