ABSTRACT
Nonalcoholic fatty liver disease (NAFLD) affects approximately 37% of US adults. The progression from nonalcoholic fatty liver with no inflammation to steatohepatitis with inflammation and progressive fibrosis is associated with substantial morbidity and mortality. The epidemic of NAFLD requires that primary care providers recognize at-risk patients and screen them. The authors review identifying individuals at risk, treatment options founded on lifestyle modification, and when to consider referring patients to a hepatologist.
Screen for NAFLD in patients with diabetes, those with 2 or more metabolic risk factors, or those with fatty liver on imaging.
The Fibrosis-4 score is a noninvasive tool using age, aspartate aminotransferase and alanine aminotransferase values, and platelet count to identify patients at risk for fibrosis.
Vibration-controlled transient elastography measures liver stiffness and helps determine the presence and severity of fibrosis.
Intensive lifestyle modification with a calorie-restricted Mediterranean diet, exercise, and weight loss is the mainstay of treatment for NAFLD.
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease in the world, affecting 25% of the world population.1 NAFLD includes nonalcoholic fatty liver (NAFL), which is fatty liver without inflammation or liver damage, and nonalcoholic steatohepatitis (NASH), which is fatty liver with inflammation or liver damage, or both. In the United States alone, NAFLD affects approximately 37% of the population,2 and the increasing incidence in the setting of obesity and the metabolic syndrome epidemic is expected to have a considerable impact on the development of cirrhosis, complications of liver disease, and liver cancer.1 NASH cirrhosis is now the leading indication for liver transplant in women, patients over age 54, and Medicare recipients.3 Patients with NAFLD are at increased risk for cardiometabolic diseases and malignancy, hence the benefit of early recognition.4
The challenge is to identify patients who have NASH and predict which patients are at the highest risk for developing fibrosis. Obesity, metabolic syndrome, and type 2 diabetes are the main risk factors for NAFLD, but the presence of other conditions such as genetic factors, sleep apnea, polycystic ovarian syndrome, and hypothyroidism also appear to play a role.5
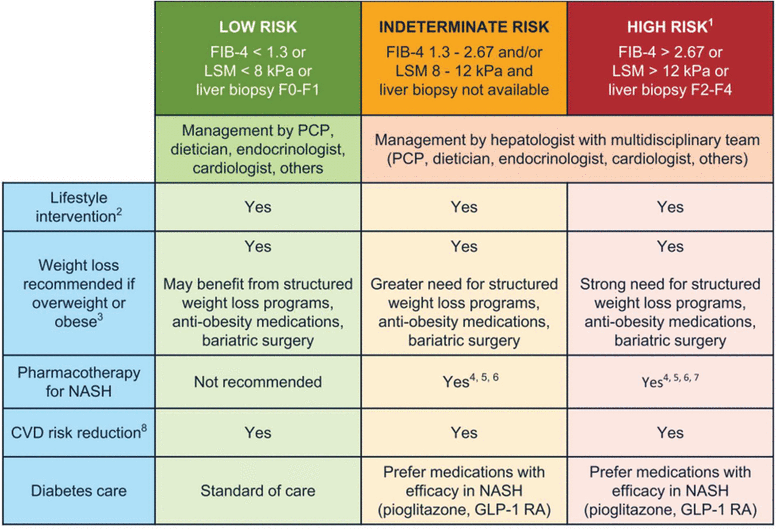
Primary care providers (PCPs) play a central role in identifying patients with NAFLD and NASH, yet gaps in knowledge may inhibit the diagnosis and management of the disease. NASH and advanced fibrosis often remain undiagnosed in the primary care setting until signs and symptoms of advanced liver disease are present. To address this need, the American Gastroenterological Association (AGA), in collaboration with other professional societies, published clinical care pathways to provide guidance to providers in screening, diagnosis, and management of NAFLD (Figures 1 and 2).2
Screening patients for NAFLD with advanced fibrosis.
1Metabolic risk factors: central obesity, high triglycerides, low high-density lipoprotein cholesterol, hypertension, prediabetes, or insulin resistance.
2For patients 65+, use FIB-4 < 2.0 as the lower cutoff. Higher cutoff does not change.
3Other NITs derived from routine laboratories can be used instead of FIB-4.
4Many online FIB-4 calculators are available such as https://www.mdcalc.com/calc/2200/fibrosis-4-fib-4-index-liver-fibrosis.
5Ultrasonography acceptable if vibration-controlled transient elastography (VCTE, FibroScan) is unavailable. Consider referral to hepatologist for patients with hepatic steatosis on ultrasonography who are indeterminate or high risk based on FIB-4.
6LSM values are for VCTE (FibroScan). Other techniques such as bidimensional shear-wave elastography or point shear-wave elastography can also be used to measure LSM. Proprietary commercially available blood NITs may be considered for patients considered indeterminate or high risk based on FIB-4 or APRI (aspartate aminotransferase-to-platelet ratio index), or where LSM is unavailable.
7Eddowes et al (Gastroenterology 2019; 156[6]:1717–1730.) used 8.2 and 12.1 kPa as cutoffs for LSM using VCTE. Validation of simple (rounded) cutoffs reported by Papatheodoridi et al (J Hepatol 2021; 74[5]:1109–1116.).
ALT = alanine aminotransferase; AST = aspartate aminotransferase; CBC = complete blood cell count; MR = magnetic resonance; NAFLD = nonalcoholic fatty liver disease
Reprinted from Gastroenterology, 161(5), Kanwal F, Shubrook JH, Adams LA, et al, Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease, 1657–1669, 2021, with permission from Elsevier.
Management of NAFLD and NASH.
1Patients with stage F4 or cirrhosis (based on biopsy, LSM values based on vibration-controlled transient elastography [VCTE, FibroScan] or > 5.0 kPa on MRE) should undergo hepatocellular carcinoma surveillance. Varices screening is recommended if LSM > 20 kPa or platelet count of < 150,000/mm3.
2All patients require regular physical activity, healthy diet, avoid excess alcohol intake.
3Weight loss recommended for cardiometabolic benefit and reversal of steatosis. Greater weight loss is often associated with more benefit, such as reversal of steatohepatitis (usually with weight loss ≥ 7%) or fibrosis (usually with weight loss ≥ 10%).
4Individualize based on further workup and efforts to confirm the diagnosis of NASH. Liver biopsy provides helpful information and should be considered when there is a diagnostic doubt, such as in patients with indeterminate, unreliable, or conflicting noninvasive assessments or as part of phase 2 or 3 clinical trials.
5No pharmacologic agent is FDA-approved for the treatment of NASH. Patients with type 2 diabetes may benefit from some diabetes medications, such as pioglitazone (Sanyal et al, N Engl J Med 2010; 362:1675–1685; Bril et al, Diabetes Care 2019; 42:1481–1488; Aithal et al, Gastroenterology 2008; 135:1176–1184; Cusi et al, Ann Intern Med 2016; 165:305–315; Belfort et al, N Engl J Med 2006; 355:2297–2307) and some GLP-1 RAs (Armstrong et al, Lancet 2016; 387:679–690; Newsome et al, N Engl J Med 2021; 384:1113–1124) that have reported histologic improvement in randomized controlled trials in patients with NASH, either with or without diabetes. Among GLP-1 RAs, semaglutide has the strongest evidence of liver histologic benefit (Newsome et al, N Engl J Med 2021; 384:1113–1124).
6Vitamin E improves steatohepatitis in patients with NASH without diabetes (Sanyal et al, N Engl J Med 2010; 362:1675–1685), with less evidence in patients with type 2 diabetes (Bril et al, Diabetes Care 2019; 42:1481–1488).
7Pharmacotherapy in patients with NASH cirrhosis is very limited and should be avoided until more data become available.
8Statins can be used safely in patients with steatohepatitis and liver fibrosis. Avoid in patients with decompensated cirrhosis.
CVD = cardiovascular disease; FDA = US Food and Drug Administration; GLP-1 RAs = glucagon-like peptide-1 receptor agonists; LSM = liver stiffness measurement; MRE = Magnetic resonance elastography; NASH = nonalcoholic steatohepatitis; PCP = primary care provider
Reprinted from Gastroenterology, 161(5), Kanwal F, Shubrook JH, Adams LA, et al, Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease, 1657–1669, 2021, with permission from Elsevier.
NAFLD AND NASH DEFINED
NAFLD encompasses a wide spectrum of conditions, ranging from simple fat infiltration in the liver (NAFL, also called hepatic steatosis), to fatty liver with inflammation (NASH), and to the development of advanced fibrosis that may progress to cirrhosis, decompensated liver disease, and hepatocellular carcinoma.
NAFL is defined by the presence of at least 5% of fat infiltration in the liver without hepatocellular injury and in the absence of other etiologic factors such as alcohol, drugs, and other chronic liver diseases. NASH involves at least 5% steatosis and inflammation with hepatocyte injury (ballooning), with or without fibrosis.6
Liver fibrosis is classified as stages F0–F4, as follows:
Stage F0–F1 (early NASH, no or mild fibrosis)
Stage F2 or higher (fibrotic NASH)
Stage F3 or higher (advanced fibrosis)
NAFLD spectrum and classification
In US adults with NAFLD, 25% will progress to NASH and 25% of patients with NASH will develop cirrhosis.11 Based on findings by Younossi et al as cited by Diehl and Day,11 liver fibrosis at the time of diagnosis is advanced in 25% of patients. It is estimated that liver fibrosis progresses by 1 stage per decade, but the rate of progression or regression varies considerably by individual.11
Patients with NAFLD have an increased overall mortality, and there is a clear association between stages of fibrosis and liver-related mortality. However, cardiovascular disease is the primary cause of death, reflecting the burden of metabolic derangement of NAFLD.6
Factors that drive progression of NAFLD include alcohol consumption and the presence of commonly associated comorbidities such as obesity, hypertension, dyslipidemia, diabetes and insulin resistance, hypothyroidism, polycystic ovarian syndrome, and obstructive sleep apnea.1
There is no consensus on the threshold of alcohol consumption that differentiates alcohol-related liver disease from NAFLD. According to Sanyal et al as cited by Cotter and Rinella,1 a common cutoff for substantial alcohol intake leading to exclusion in NASH clinical trials is more than 21 drinks weekly for men and more than 14 drinks weekly for women. The National Institute on Alcohol Abuse and Alcoholism as cited by Cotter and Rinella1 defines a standard drink as containing 14 g of alcohol.
The effect of alcohol on NAFLD progression is difficult to assess because of inaccurate reporting of alcohol consumption and genetic differences in susceptibility to alcohol-related liver injury. It is best to assume that there is no safe amount of alcohol consumption for patients with NAFLD.1 Given the lack of precise definition of significant alcohol consumption in patients suspected of having NAFLD, the concept of the term “metabolic dysfunction-associated fatty liver disease” (MAFLD) has been proposed.12,13 MAFLD, which encompasses the previously discussed definition of NAFLD, is more inclusive than NAFLD as it does not exclude excessive alcohol usage in its definition.12
It is worth noting that contrary to other liver diseases in which hepatocellular carcinoma (HCC) develops from cirrhosis, patients with NAFLD may develop HCC without the presence of cirrhosis. In a population-based study of medical records from 26 major integrated US healthcare systems, out of 392,000 NAFLD patients identified, 1,110 had a diagnosis of HCC, and of those, 170 (15.3%) did not have cirrhosis. Risk factors for development of HCC in the noncirrhotic patients were identified as older male sex, smoking history, diabetes, and elevated alanine aminotransferase.14
Patients with NAFLD who are diagnosed with HCC are typically older with higher extrahepatic comorbidities and a lower prevalence of cirrhosis than patients with HCC due to viral or alcohol-related liver pathology. The occurrence of HCC in the absence of liver cirrhosis poses a challenge for surveillance.15 Liver fibrosis progresses over time and typically remains asymptomatic until patients present with decompensated cirrhosis or are diagnosed with HCC, at which time the opportunity for curative treatment decreases.16,17
SCREENING
In a primary care setting, NASH and advanced fibrosis are often undiagnosed until signs and symptoms of advanced liver disease are present. As such, PCPs are on the front line of identifying patients with NAFLD and stratifying patients at risk for developing advanced fibrosis in order to provide optimal management and referral. Different screening algorithms have been proposed to facilitate the delivery of care to patients and to optimize appropriate referrals to hepatology.18–21 The AGA recommends screening for NAFLD with fibrosis (Figure 1)2 in patients with the following:
2 or more metabolic risk factors (central obesity, triglycerides ≥ 150 mg/dL, high-density lipoprotein < 40 mg/dL in men or < mg/dL 50 in women, hypertension, prediabetes)
Type 2 diabetes mellitus
Incidental findings of fatty liver or elevated liver enzymes.
Screening for these high-risk individuals should include assessment for excessive alcohol use (> 21 drinks/week for men, > 14 drinks/week for women) and basic laboratory studies, including complete blood cell count and liver enzymes.2
While NAFLD often presents with abnormal liver enzyme levels, the levels may be normal even in patients with advanced fibrosis. In a systematic review and meta-analysis of 4,084 patients, the alanine aminotransferase was normal in 25% of patients with NAFLD and 19% of patients with NASH.22 The initial assessment of elevated liver enzymes starts with the exclusion of alternative or coexisting causes of liver or biliary diseases. This is best achieved by obtaining a detailed alcohol-intake history, evaluating for clinical signs of advanced liver disease, testing for hepatitis C, and consideration of testing for hepatitis B, autoantibodies (antinuclear, antimitochondrial, anti-smooth muscle), ferritin, immunoglobulins, and alpha-1 antitrypsin. Liver imaging to evaluate for mass lesions should also be performed.2
DIAGNOSIS
Fatty liver is typically detected on imaging studies such as ultrasonography or other advanced imaging. There is no laboratory test or imaging study that can conclusively diagnose NASH. The gold standard for NASH diagnosis and differentiation from NAFL is liver biopsy, the utility of which is limited due to invasiveness, risk of complications, patient acceptability, sampling variability, and cost.
Given these limitations and the high prevalence of NAFLD, it is important for PCPs to feel comfortable using noninvasive tools to assess for NASH, advanced fibrosis, and cirrhosis.23 Noninvasive testing includes the use of serum biomarkers and imaging studies.
Scoring systems
Scoring systems that utilize simple clinical and laboratory variables to assess the likelihood of advanced liver fibrosis include the Aspartate Transaminase Platelet Ratio Index, the NAFLD Fibrosis Score, and the Fibrosis-4 (FIB-4) score.23 The FIB-4 score utilizes age, platelet count, aspartate aminotransferase, and alanine aminotransferase for evaluation of advanced fibrosis. It has been validated in patients with hepatitis C and human immunodeficiency virus coinfection to assess the need for biopsy and has more recently been used in patients with NAFLD.24 In a study of 541 adults with NAFLD, a FIB-4 cutoff score of 1.3 or less had a 90% negative predictive value, while a cutoff of at least 2.67 conferred an 80% positive predictive value for advanced fibrosis.25
Imaging
Ultrasonography is more effective at detecting steatosis in patients with moderate to severe steatosis (greater than 20% to 30%) but less effective in patients with mild steatosis (< 20%).7 Therefore, it is important to stratify a patient’s risk of steatosis even if the ultrasound does not show steatosis.
Liver elastography can be used with both ultrasonography and magnetic resonance imaging. Transient elastography with the FibroScan device uses pulse-echo ultrasound waves to evaluate liver stiffness as an indirect indicator of the presence or absence of advanced fibrosis and steatosis. It can be used in most patients, except in those with severe obesity. Magnetic resonance elastography is very sensitive at diagnosing steatosis and fibrosis, but it is expensive and not widely available.23
Other approaches to risk stratification
Noninvasive markers for advanced fibrosis and the development of novel pharmacologic agents that affect natural progression of advanced fibrosis26,27 present an opportunity for PCPs to identify patients at high risk. Although there is no preferred approach to risk stratification, the guiding principle is to rule out advanced fibrosis using simple, noninvasive technology such as FIB-4 scoring, followed by transient elastography in patients at intermediate or high risk. Currently, FIB-4 scoring is one of the best noninvasive biomarkers, and its performance is enhanced by combining it with elastography in a sequential manner. The combination was found to be cost-effective in addition to providing high diagnostic accuracy,18,28 and it represents an opportunity for PCPs to develop a partnership with a gastroenterology or hepatology practice and avoid unnecessary referrals.
MANAGEMENT OF NAFLD AND NASH: LIFESTYLE MODIFICATION
Intensive lifestyle modification including weight loss, diet, and exercise is the first-line intervention and the only approved therapeutic approach for treating NAFLD. Given the considerable challenges of lifestyle modifications, a multidisciplinary team approach that includes a physician, dietitian, psychologist, and exercise physiologist is optimal. When a multidisciplinary team is not available, physician guidance can affect outcomes, as several studies have shown that physicians play an important role in motivating patients to lose weight with diet and exercise recommendations. They can also provide regular follow-up care.29
Figure 22 is a clinical care pathway for the management of NAFLD and NASH by risk of fibrosis.
Weight loss goals
Weight loss of 5% or more of total weight can decrease liver steatosis, loss of 7% or more can lead to resolution of NASH, and loss of 10% or more can lead to fibrosis regression or lack of progression (Table 1).7–10 In a prospective study of 293 patients with histologically defined NASH encouraged to follow lifestyle modification for weight loss over 52 weeks, there was resolution of NASH in 90% and regression of fibrosis in 45% of patients who lost 10% or more of their baseline body weight.30
In order to achieve substantial weight loss, daily calories should not exceed 1,200 kcal for women and 1,500 kcal for men. A low-calorie diet should be prescribed, even for patients with lean NAFLD (body mass index ≤ 25 kg/m2 in non-Asian or ≤ 23 kg/m2 in Asian patients), targeting a weight loss of 3% to 5%, given the histologic benefits for steatosis and NASH.8
Weight loss medications
Antiobesity medications, ideally in the setting of a structured weight-loss program, should be considered in the appropriate patients. A detailed discussion of antiobesity medications is beyond the scope of this article, but glucagon-like peptide-1 (GLP-1) agonists such as liraglutide or semaglutide may be good options,31,32 as discussed below (see “Drug therapy.”)
Diet
Most experts recommend the Mediterranean diet for patients with NAFLD. This diet is rich in olive oil, fish, nuts, whole grains, fruits, and vegetables. It has shown superiority in long-term weight loss compared with low-fat diets and improves metabolic derangement and steatosis even without weight loss.33 Refined carbohydrates and alcohol should be avoided. Intake of refined carbohydrates is linked to increased systemic inflammation, which worsens NAFLD.34
Patient acceptance of dietary intervention is challenging because of habits, culture, and ethnicity,35 but it is important to implement strategies to avoid relapse of weight gain. Ideally, dietary intervention is applied to the entire household to improve adherence. Lifestyle intervention is less effective in resolving NASH in elderly patients, patients with type 2 diabetes, and patients with more severe histologic activity on liver biopsy.29
Exercise
Exercise, even without weight loss, can lead to a 20% to 30% reduction of intrahepatic lipids.29 This occurs through various pathways, including improved peripheral insulin resistance and a decrease in delivery of fatty acids to the liver.36 A behavioral assessment for eating disorders and underlying psychiatric disorders such as depression can be valuable. Barriers to engagement in exercise should be evaluated, with practical solutions discussed with the patient.34
SURGICAL AND PHARMACOLOGIC OPTIONS
Bariatric procedures
Bariatric surgery or, more appropriately, metabolic surgery,37 has been shown to substantially improve NASH in patients with obesity as reported in a prospective study of 109 patients, in which 70 patients (85%) had resolution of NASH after bariatric surgery.9 In a recent retrospective analysis of 196 patients who underwent bariatric surgery, active steatohepatitis was successfully reversed with 70% of patients showing fibrosis regression of 1 or more stages, but advanced fibrosis persisted in 47% of patients.10 Endoscopic bariatric procedures (eg, intragastric balloon, transpyloric shuttle, gastric reduction or plication, duodenojejunal bypass liner, and dual-path enteral bypass magnets) have also been effective in NAFLD by both weight loss-dependent and weight loss-independent pathways.38
Drug therapy
No medications for the treatment of NAFLD have been approved by the US Food and Drug Administration (FDA), but many pharmacologic agents are being evaluated for the treatment of NASH.39 The mainstay of treatment for NAFLD remains weight loss, exercise, and treating metabolic comorbidities such as diabetes and dyslipidemia. Management of comorbidities presents PCPs with an opportunity to prescribe medications that may have a positive effect on reducing fibrosis, such as pioglitazone, GLP-1 receptor agonists, and sodium-glucose co-transporter-2 inhibitors.
Pioglitazone has been shown to improve insulin sensitivity, lower liver enzyme levels, and reduce NASH regardless of the presence of type 2 diabetes, but there are many adverse effects including weight gain.6,26 Pioglitazone 30 mg once daily improves hepatic steatosis and inflammation, but 45 mg once daily is needed to improve fibrosis.26 Given the improvement on liver histology, the American Association for the Study of Liver Diseases practice guidelines indicate that pioglitazone may be used in patients with biopsy-proven NASH. However, the risks and benefits should be considered and discussed with patients before initiation of therapy.6
GLP-1 receptor agonists liraglutide and semaglutide are currently being studied as treatment for NASH. The results appear promising, with improvement of liver enzyme levels, liver histology, and insulin resistance, but additional studies are needed to evaluate routine use for treatment of NASH.31,32 Although these 2 medications are not yet FDA-approved for the treatment of NASH, they could be considered for treatment of diabetes or obesity in patients with NAFLD, as both medications have FDA indications for diabetes and obesity.
Sodium-glucose cotransporter 2 inhibitors are currently being studied in NAFLD. Trials include the Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and nonalcoholic Fatty Liver Disease trial40 and the Dapagliflozin Efficacy and Action in NASH study.41 Early results indicate that empagliflozin and dapagliflozin reduce steatosis in patients with type 2 diabetes,41,42 and dapagliflozin may also reduce liver fibrosis. However, this finding was only seen in patients with substantial liver fibrosis, and it is not clear if weight loss due to the medication caused the improvement.41
Metformin has been shown to improve insulin resistance and lower liver enzymes in patients with NAFLD.43,44 However, it does not improve histology.45 In a meta-analysis of 4 high-quality randomized controlled trials, Musso et al46 found no improvement in liver enzymes or histology in individuals with NASH treated with metformin plus lifestyle intervention compared with those treated with lifestyle intervention alone—independent of dose, treatment duration, or presence of diabetes. Because metformin has not been shown to improve fibrosis, the American Association for the Study of Liver Diseases does not recommend this medication for the treatment of NASH.6
Vitamin E, coffee, and herbals
In patients without diabetes, vitamin E 800 IU daily has been shown to improve NASH but does not have a considerable effect on fibrosis.47
Moderate caffeine intake has been associated with a lower risk of all-cause mortality as evidenced in an analysis of a large group of adults in the National Health and Nutrition Examination Survey 1999 to 2014.48 A recent meta-analysis of 11 epidemiologic studies showed that regular coffee consumption has a favorable effect on NAFLD49: individuals who drink coffee regularly had a 23% decreased risk of development of NAFLD compared with those who did not regularly drink coffee. Individuals with established NAFLD who drank coffee daily had a 32% reduced risk of developing fibrosis. Setiawan et al and Wadhawan et al as cited in Hayat et al49 reported that drinking more than 2 cups of coffee a day was associated with a lower risk of liver fibrosis, cirrhosis, and hepatocellular carcinoma. The proposed mechanism for this decrease in liver injury associated with drinking coffee is the antioxidant effects of caffeine, as well as several other components found in coffee.49
Silymarin, an extract of milk thistle, was reported to reduce fibrosis without improvement in steatosis or inflammation, though larger studies are needed.50 Resveratrol, a chemical found in red wine, may in conjunction with lifestyle modification improve inflammation in patients with NAFLD, though the benefits in NASH are inconsistent.51
CONCLUSION
In this era of a global epidemic of NAFLD, PCPs play an essential role in identifying patients with NAFLD and in screening them for advanced fibrosis using noninvasive techniques. The screening and management algorithms proposed by the AGA provide an opportunity to develop partnerships with gastroenterology or hepatology practices and avoid unnecessary referrals. There is no FDA-approved pharmacotherapy for NASH. Intensive lifestyle modification to manage weight, diet, and physical activity is the only approved therapy.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Acknowledgment
The Scientific Publications staff at Mayo Clinic provided copyediting support.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.