ABSTRACT
Cutaneous findings can be clues to diagnosis and infection severity in viral illnesses, including COVID-19. The authors provide an update on the diagnostic and prognostic value of the 5 most common cutaneous abnormalities associated with COVID-19 in adult patients: morbilliform rash, urticaria, vesicles, pseudo-chilblains, and vaso-occlusive lesions.
The common cutaneous abnormalities that occur in COVID-19 patients were recognized early in the pandemic, and evidence concerning their pathogenesis and clinical relevance continues to accumulate.
Urticarial and vesicular eruptions may precede other COVID-19-associated symptoms and, along with morbilliform rashes, are typically associated with overall high survival rates.
The association of pseudo-chilblains with COVID-19 remains controversial, and no definitive evidence linking them to SARS-CoV-2 infection has been reported.
The most worrisome manifestations are vaso-occlusive skin lesions, which most often occur in hospitalized patients with COVID-19 and are associated with a poorer prognosis than other skin lesions.
As experience with caring for patients with COVID-19 has accumulated since the onset of the pandemic, so has our understanding of its associated cutaneous manifestations and their clinical implications.
It is beneficial to watch for cutaneous manifestations of COVID-19, both in and out of the hospital. For example, a study of more than 330,000 community-based patients in the United Kingdom1 found that patient-reported skin rash was associated with positive COVID-19 testing and was more predictive than fever. Additionally, an analysis of 296 hospitalized patients with COVID-19 in the United States2 found that mucocutaneous findings were associated with the need for mechanical ventilation, even when adjusted for age, body mass index, and comorbidities.
COVID-19-associated cutaneous abnormalities are often grouped into 5 major categories (Table 1)3:
Morbilliform rash (containing macules and papules, resembling measles)
Urticaria (itchy red welts)
Vesicles (small blisters)
Pseudo-chilblains (also known as “COVID toes,” painful inflammation of the digits in response to cold)
Vaso-occlusive lesions (due to thrombosis and occlusion of small arteries, with subsequent ischemia).
Major categories of cutaneous eruptions in COVID-19
MORBILLIFORM RASH: THE MOST COMMON SKIN MANIFESTATION
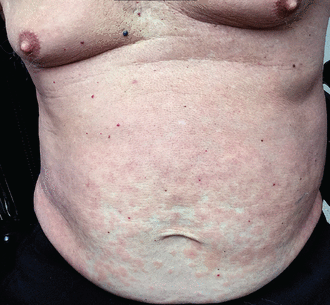
Morbilliform eruptions are common in many viral illnesses and were reported in patients with COVID-19 early in the pandemic.4,5 International registry data indicate that morbilliform eruptions are the most common cutaneous manifestation in patients with laboratory-confirmed COVID-19.6 Typical clinical features include a generalized, symmetric maculopapular rash with pruritus (Figure 1).7
Morbilliform rash in COVID-19. A 77-year-old man was hospitalized with COVID-19 and developed bilateral pneumonia and acute hypoxic respiratory failure. Four days after discharge, while continuing to have low-grade fevers, he developed pink papules confluent over the trunk and extremities consistent with a morbilliform eruption.
Patients with COVID-19-associated morbilliform eruptions have an excellent prognosis, with survival rates of 96.9%8 to 97.5%.3
URTICARIA CAN BE THE FIRST SIGN OF COVID-19
Urticaria is also common in COVID-19. The clinical features do not appear to differ from those of idiopathic urticaria and typically consist of generalized pruritic wheals.9,10 On average, urticaria lasts less than 1 week11 and is associated with relatively mild disease and survival rates of 97.8%6 to 98.2%.3
Histologic features also mimic those of idiopathic urticaria and thus limit the value of skin biopsy.9,10 However, urticarial vasculitis has been described in association with COVID-19, suggesting that biopsy should be considered in patients with persistent urticarial plaques with associated purpura.12
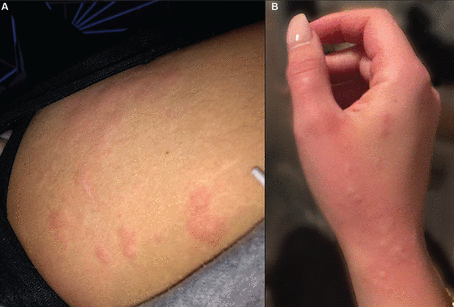
Interestingly, in a systematic review of 895 patients with COVID-19,13 105 (12%) had urticarial lesions, and in 17 (16%) of these 105 the urticaria began before the onset of the other COVID-19 symptoms, suggesting that it can be a clue to diagnosis in appropriate clinical settings and can help guide early testing (Figure 2).13 Additionally, an analysis of 200 patients with COVID-19 with cutaneous manifestations14 found a significant association between urticaria and gastrointestinal symptoms, which could assist clinicians in their anticipatory management.
Urticarial lesions preceding COVID-19 diagnosis. A 21-year-old woman with no known previous skin problems developed urticarial lesions in various locations, including the thighs (A) and hands (B) several days before testing positive for COVID-19 as part of a routine screening protocol. She subsequently experienced systemic symptoms including palpitations, cough, fatigue, and loss of taste and smell, but was able to be managed on an outpatient basis with supportive care.
VESICLES CAN ALSO BE THE FIRST SIGN OF COVID-19
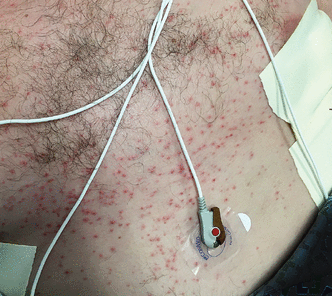
Initially described as “varicella-like,”15 vesicular eruptions in COVID-19 have been described in both localized and diffuse distributions. The localized pattern is characterized by monomorphic vesicles in the same stage of evolution that are confined to the trunk (Figure 3). But the diffuse pattern may be more common. A cohort study16 reported that it accounted for 18 (75%) of 24 cases. The diffuse pattern consists of polymorphic papules, vesicles, and pustules that develop simultaneously on the trunk and spread distally, sometimes involving the palms and soles. Lesions tend to resolve after about 8 days without scarring.15
Vesicular eruption in COVID-19. A 52-year-old man developed a vesiculopustular eruption on his trunk during hospitalization for COVID-19 requiring intensive care unit admission and mechanical ventilation for acute respiratory failure due to respiratory distress syndrome. He recovered and was eventually discharged.
Lesional skin biopsies reveal histologic features consistent with viral exanthems, namely vacuolar degeneration of the basal epidermal layer with occasional dyskeratotic keratinocytes and superficial dermal inflammation.15 However, some reports describe prominent keratinocyte acantholysis contributing to formation of intraepidermal vesicles, which is a relatively unusual histologic finding.17 Additionally, while there have been reports of SARS-CoV-2 spike proteins detected with immunohistochemistry in sweat glands and dermal endothelial cells in skin biopsies from COVID-19 patients, 2 studies of COVID-19-associated vesicular rashes detected no SARS-CoV-2 in vesicular fluid by reverse transcriptase polymerase chain reaction testing.16–18
Like urticaria, vesicular eruptions were also commonly noted before other COVID-19 symptoms (in 8.5%3 to 15%13 of cases of COVID-19-associated urticaria) in multiple studies, and therefore may similarly provide an indication for COVID-19 testing and isolation in the appropriate clinical context.3,14,19 Additionally, a systematic review8 reported a possible link between vesicular eruptions and neurologic symptoms including headache, dysgeusia, irritability, and confusion. Like those with morbilliform rash or urticaria, patients with COVID-19 with vesicular eruptions have high survival rates (96.1%3 to 96.6%8).
PSEUDO-CHILBLAINS: LINK TO COVID-19 DEBATED
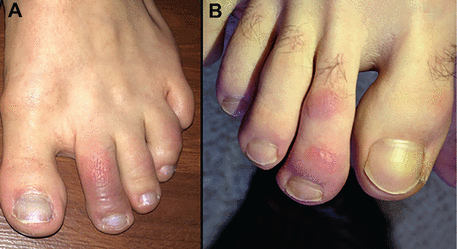
Although pernio-like acral lesions (Figure 4) were the first cutaneous manifestations to generate significant attention, whether they are truly linked to COVID-19 has been debated.
Pseudo-chilblains (“COVID toes”). (A) A 24-year-old woman developed painful erythematous and violaceous macules involving her dorsal toes after testing positive for COVID-19. She had no other symptoms. (B) A young adult man sought care in a telemedicine encounter after developing painful, erythematous papules on his toes. His eventual COVID-19 status is unknown.
Challenging the link are strikingly low rates of positive COVID-19 testing in affected patients, as well as results of several studies20–24 that suggest these lesions are most consistent with typical perniosis, with an increased incidence related to changes in daily routine (eg, quarantining, working from home) during the pandemic rather than infection with SARS-CoV-2. Additionally, a systematic review8 found that pre-existing rheumatologic conditions were more common in patients with presumed COVID-19-related pernio-like lesions, raising the possibility that underlying diagnoses contributed to development of the acral lesions.
However, proponents of the association with COVID-19 point to “outbreaks” of chilblain-like lesions corresponding to COVID-19 waves and propose that an efficient, type I interferon-driven antiviral response could induce pernio-like lesions and suppress both symptoms and confirmatory testing.25–29 Interestingly, information is accumulating about pernio-like lesions in “long-hauler” patients, with a significant association reported between persistent cutaneous and extracutaneous symptoms.30–32
While the debate continues, if these lesions are truly a COVID-19 manifestation, they are fortunately associated with high survival rates (96.4%6 to 98.7%3) and few or no systemic symptoms.33,34
VASO-OCCLUSIVE LESIONS ARE ASSOCIATED WITH HIGHER RISK
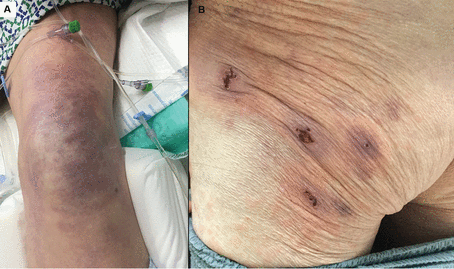
Vaso-occlusive lesions (Figure 5) have been reported in patients with COVID-19 with varied clinical presentations, including fixed livedo racemosa, retiform purpura, and acral ischemia, which may be clinically confused with COVID toes.19 These lesions are most commonly seen in hospitalized patients with moderate to severe COVID-1919 and are associated with higher risks of severe pneumonia and intensive care unit admission and relatively low survival rates (78.9%3 to 81.8%8).
Vaso-occlusive lesions in COVID-19. (A) A 62-year-old man with COVID-19 developed an irregular, mottled, purpuric patch on his knee extending onto his thigh during an extended hospitalization complicated by septic shock and acute respiratory failure requiring mechanical ventilation. He died of his illness 3.5 weeks after admission. (B) A 77-year-old man (also described in Figure 1) developed purpuric patches with central hemorrhagic crusts on the left buttock shortly after hospitalization for COVID-19.
Similar patterns of microvascular thrombosis have been found in skin biopsies and pulmonary tissue of COVID-19 patients with vaso-occlusive cutaneous lesions, suggesting that this manifestation could be a marker of systemic microvascular injury.35 Additionally, systemic thrombotic events including deep vein thrombosis and pulmonary embolism have been reported in patients with retiform and necrotic lesions, with rates as high as 64%.6,36 Whether early recognition of these lesions can prompt treatment decisions that decrease systemic thrombotic events or increase overall survival requires further research.
OTHER CUTANEOUS FINDINGS
Other cutaneous findings that have been reported with COVID-194,7,37–39 include oral lesions; reactivation of viral infections; rash resembling symmetrical drug-related intertriginous and flexural exanthema; small-vessel vasculitis; cutaneous hyperesthesia; papulosquamous eruptions; and erythema nodosum-like lesions.
Oral lesions. A study of 666 patients40 reported various oral mucosal findings in 78 (26%) of 304 patients who had mucocutaneous manifestations, and the authors hypothesized that lesions in the mouth may be under reported due to contact precautions and assisted ventilation that limits examination of the oral mucosa.40
Reactivation of herpes simplex virus (HSV) and varicella-zoster virus (VZV) infections has been reported in conjunction with COVID-19 infection. A cross-sectional study of nearly 900 patients with COVID-19 found a significantly higher prevalence of HSV-1 and VZV than in the hospital population, even when adjustments were made for numerous comorbidities.41 Some reports suggest that HSV reactivation may be associated with more severe COVID-19 infection, including acute respiratory distress syndrome and viremia,42,43 but the prognostic implications of treating these reactivations has not yet been robustly investigated.
DISCLOSURES
Dr. Fernandez reports consulting for Abbvie Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Mallinckrodt, Novartis, and UCB; teaching and speaking (non-promotional) for Abbvie Pharmaceuticals, Kyowa Kirin, Mallinckrodt, and Novartis; research/independent contracting for Abbvie Pharmaceuticals, Mallinckrodt, Novartis, and Pfizer; and work as advisor or review panel participant for Abbvie Pharmaceuticals. Dr. Polly reports no relevant financial relationships which, in the context of her contributions, could be perceived as a potential conflict of interest.
Acknowledgment
The authors would like to thank Janine Sot, MBA, for her help and expertise in preparing the figures in this article.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.