A 62-year-old woman presented to the emergency department after suddenly becoming short of breath at rest. Over the past 24 hours she had also noticed a decline in her home spirometry values, and dry cough and fatigue. She had not experienced any fever, chills, weight loss, lymphadenopathy, rhinorrhea, chest pain, or palpitations. She had not traveled recently and had not been in contact with anyone who was sick.
Six years earlier, she had undergone bilateral lung transplantation for chronic respiratory failure due to usual interstitial pneumonitis. Of note, prior to transplant, both this patient and the donor had tested positive for Epstein-Barr virus and cytomegalovirus.
Her medications included low-dose aspirin, trimethoprim-sulfamethoxazole, azithromycin, and an immunosuppressive regimen of tacrolimus, prednisone, and mycophenolate. She said she took her medications faithfully and did not use tobacco, electronic cigarettes, alcohol, or recreational drugs, including intravenous ones.
PHYSICAL EXAMINATION NORMAL, BUT AN OPACITY IN HER LUNG
On admission, the patient was hemodynamically stable and had an oxygen saturation of 97% while breathing room air. She did not appear to be in acute distress and could converse in full sentences without difficulty.
Her heart, lungs, and abdomen were normal on examination, with no rales, rhonchi, wheezes, or decreased breath sounds. While in the emergency department, her temperature went up to 38.1°C (100.6°F) without any other significant changes in her vital signs. She was given acetaminophen, and her temperature came back down.
Initial blood test results were as follows:
White blood cell count 10.6 × 109/L (reference range 3.4–9.6); a differential count was not done
Procalcitonin below the limit of detection, ie, less than 0.06 ng/mL
Tacrolimus 4.3 ng/mL (reference range 5.0–15.0, goal 6.0–8.0)
Human leukocyte antigen class I and II antibodies, negative
Aspergillus (galactomannan) antigen, negative
Epstein-Barr virus and cytomegalovirus viral load undetectable.
Arterial blood gasses were not measured, as the patient was clinically stable without tachypnea or oxygen desaturation. All other laboratory results were within normal limits.
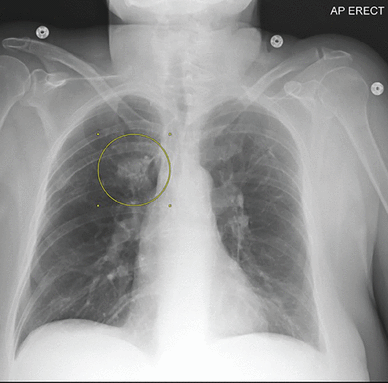
Chest radiography revealed a new, ill-defined opacity superior to the right hilum (Figure 1).
Chest radiograph on admission showing a new opacity superior to the right hilum (circle).
Although her seemingly benign symptoms could have been due to a self-limiting illness, in view of her immunosuppressed state, she was admitted for further workup and management.
DIFFERENTIAL DIAGNOSIS OF ACUTE DYSPNEA IS BROAD
1. What is the most likely cause of this patient’s symptoms?
Respiratory tract infection
Pulmonary embolism
Lung transplant rejection and dysfunction
Posttransplant lymphoproliferative disorder
For patients presenting with acute onset of dyspnea and cough, the differential diagnosis is relatively broad and includes pulmonary embolism, myocardial infarction, and respiratory tract infections such as pneumonia or bronchitis. However, in this patient who has a lung transplant, it is important to also consider primary lung graft dysfunction, acute lung transplant rejection, and chronic lung allograft dysfunction, specifically bronchiolitis obliterans syndrome and restrictive allograft syndrome. And in view of her immunosuppressed state, it is also important to consider opportunistic infections, lymphoma, and iatrogenic injury due to the toxic effects of the immunosuppressive regimen.
Respiratory tract infection
Respiratory tract infection can include pneumonia, bronchitis, or bronchiolitis. It must be strongly suspected in immunocompromised patients, since they are prone to rapid deterioration and are at high risk of infection from a broad array of pathogens, many of which may not affect immunocompetent patients.
The most common pathogens, in order of greatest incidence, include bacteria (eg, Staphylococcus aureus, Pseudomonas species, Enterobacteriaceae, enterococci, Haemophilus influenzae, mycobacteria), viruses (eg, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, influenza, respiratory syncytial virus, adenovirus, parainfluenza virus, coronavirus), and fungi (eg, Pneumocystis jirovecii, Aspergillus).1
Respiratory tract infection is the most likely diagnosis in our immunocompromised patient with dyspnea, cough, and fever with leukocytosis and chest radiography positive for a perihilar opacity.
Pulmonary embolism
Pulmonary embolism classically presents with pleuritic chest pain and dyspnea at rest with a physical examination notable for tachycardia, hypoxia, or both.2 Although our patient’s presentation with dyspnea at rest, cough, fever, and leukocytosis could be explained by pulmonary embolism, her pretest probability of pulmonary embolism was zero based on the absence of hereditary and acquired risk factors (eg, clinical signs or symptoms of deep vein thrombosis, hemoptysis, immobilization). Therefore, additional evaluation for pulmonary embolism was not warranted.
Lung transplant rejection or dysfunction
Transplant rejection or dysfunction should be high on the list of differential diagnoses when a recipient presents with new or worsening respiratory symptoms or a decline in spirometric values. Determining how long after transplant the symptoms began can help establish the type of rejection.
Primary lung graft dysfunction is not a consideration in our patient, as it presents within 72 hours of transplant with hypoxemia and evidence of diffuse alveolar infiltrates on chest radiography.3
Acute rejection typically occurs within the first 6 months after lung transplantation and can be subdivided into cellular and antibody-mediated rejection.4 Our patient received her lung transplant 6 years ago and has no history of rejection, making this diagnosis less likely, even though her serum tacrolimus level was subtherapeutic on admission. The absence of serum human leukocyte antigen antibodies during initial laboratory testing was also reassuring.
Chronic lung allograft dysfunction is a leading cause of long-term morbidity and mortality in lung transplant recipients and is a major reason the 5-year survival rate is only about 55%.5,6 It typically occurs more than 6 months after lung transplant and is characterized by an obstructive (ie, bronchiolitis obliterans syndrome) or restrictive phenotype.7
Our patient reported a decrease in her home spirometry values, but it was an acute decrease, occurring over less than 3 weeks, making chronic lung allograft dysfunction less likely. Also, our patient has been taking azithromycin prophylactically, which has demonstrated effectiveness in preventing bronchiolitis obliterans syndrome.5,7 However, if other possible causes of her acute symptoms are excluded, then additional evaluation may be warranted.
Posttransplant lymphoproliferative disorder occurs in 3% to 10% of lung transplant recipients, who are the group of transplant recipients with the second highest incidence rate of this disorder (after multiorgan and intestinal transplant recipients).8 Its presentation is nonspecific, but it should be considered in lung transplant recipients presenting with a mononucleosis-like syndrome (fever, malaise, tonsillitis, pharyngitis) or fever of unknown origin.9 It is strongly associated with Epstein-Barr virus.
Our patient had no constitutional symptoms such as weight loss or fever, no asymmetric lymphadenopathy on examination, and no detectable Epstein-Barr virus in peripheral blood, making this diagnosis less likely. However, as noted, both the patient and the donor were seropositive for Epstein-Barr virus before transplant.
THE NEXT STEP
2. What should be the next step in this patient’s management?
Obtain a viral respiratory pathogen panel, including urine, sputum, and blood cultures
Empirically start broad-spectrum intravenous antimicrobials
Perform bronchoscopy with bronchoalveolar lavage and transbronchial biopsy
Obtain serial chest radiographs and treat her symptoms only
Because infection is very strongly suspected in our patient, the next step should be a viral respiratory pathogen panel, sputum Gram stain and cultures, and blood cultures, and empirical antimicrobial therapy should be started.
Identifying a culprit microbe early will help in forming an appropriate treatment plan.9 Although the cost, appropriateness, and usefulness of each diagnostic test must be carefully weighed, these tests are necessary in an immunocompromised patient. Our patient’s presentation also coincided with the ongoing COVID-19 pandemic, so based on the US Centers for Disease Control and Prevention testing criteria,10 she was tested for SARS-CoV-19 infection.
Starting broad-spectrum intravenous antimicrobials empirically is recommended before the organism responsible for the suspected infection is identified in patients at high risk of severe infection, such as solid-organ transplant recipients. These should be started right away and not delayed for specimen collection if the patient is hemodynamically unstable.
The likely type of infection (bacterial, viral, or fungal) and therefore the microorganisms to consider when selecting empiric antimicrobial coverage depends on the time elapsed since the transplant procedure: the postsurgical phase (< 4 weeks), the period of maximum immunosuppression (1 to 6–12 months), or beyond 6 to 12 months.11,12
It is also important to consider environmental exposures (eg, recent travel); bacterial colonization history, including Pseudomonas, methicillin-resistant S aureus, or multidrug-resistant organisms, particularly in patients with cystic fibrosis; and whether the patient has been taking antimicrobials prophylactically.11
We selected antimicrobials for our patient to cover community-acquired bacterial pathogens, the most common causes of infection in the period beyond 6 months after transplant. We started intravenous cefepime and vancomycin because they have a broad spectrum of antimicrobial activity and because our patient had a history of allergy to fluoroquinolones and penicillin. Prophylactic trimethoprim-sulfamethoxazole for P jirovecii pneumonia and azithromycin for bronchiolitis obliterans syndrome were also continued.
Bronchoscopy with bronchoalveolar lavage and biopsy is invasive but is a reasonable next step in a lung transplant recipient in whom infection or rejection is a concern if the etiology or causative agent or agents remain unclear in the next 24 to 48 hours from the time of initial testing.1
Our patient had undergone surveillance bronchoscopy with bronchoalveolar lavage after receiving her lung transplant, and the findings were unremarkable. During her hospital stay (lasting 4 days), her oxygen requirements remained unchanged and her clinical condition improved, even though her cough changed from nonproductive to productive. We still strongly suspected infection or rejection, or both, despite unremarkable Gram stain and culture results for blood and sputum, including a negative result for COVID-19, and we therefore went ahead with bronchoscopy with bronchoalveolar lavage and transbronchial biopsy.
Serial chest radiographs can help find an explanation for new or worsening signs and symptoms such as a productive cough,13 which our patient developed during her hospital course. Certain findings on imaging can suggest particular types of infectious or noninfectious etiologies.
In our patient, the acute finding of a focal air-space opacity may suggest a bacterial cause. If this finding is subacute or chronic, resistant bacterial infection, fungi, Nocardia, and mycobacterial infection may be more likely, including atypical P jirovecii pneumonia or bronchiolitis obliterans organizing pneumonia.1
High-resolution computed tomography after chest radiography can be useful if the radiographs appear normal.1 In cases of suspected acute rejection, it typically shows ground-glass opacities, interlobular septal thickening, nodules, consolidation, and volume loss, whereas in patients with suspected chronic rejection, such as bronchiolitis obliterans syndrome, it may show bronchial dilation, bronchial wall-thickening, and mosaic attenuation primarily in the lower lobes.14 High-resolution computed tomography was not deemed necessary in view of our patient’s abnormal results on radiography, and the inpatient team decided that bronchoscopy with bronchoalveolar lavage and transbronchial biopsy would be more definitive in diagnosing her illness.
However, most findings on imaging studies are nonspecific, and therefore microbial identification and histopathologic analysis would be more useful. Also, treating the symptoms alone would be inadequate in our immunocompromised patient.
HOSPITAL COURSE
In our patient, bronchoscopy was overall unremarkable, with minimal secretions noted. Bronchoalveolar lavage samples were obtained near the site of the focal opacity identified on imaging and tested for the following:
Aspergillus antigen
Bacteria, including Legionella and methicillin-resistant S aureus, by Gram stain and culture
Fungi by a smear and culture
Mycobacteria by acid-fast smear and culture
P jirovecii by a smear
Bordetella pertussis, B parapertussis, B bronchiseptica, and B holmesii
Viral respiratory pathogen panel with a polymerase chain reaction assay.
The viral pathogen panel included the following organisms:
Adenovirus
Cytomegalovirus by polymerase chain reaction
Herpes simplex virus 1 and 2 by polymerase chain reaction
Human metapneumovirus (HMPV)
Influenza AH1, AH3, and B
Parainfluenza virus 1, 2, 3, and 4
Respiratory syncytial virus A and B
Rhinovirus.
Testing did not include polymerase chain reaction assays for Chlamydia pneumoniae or Mycoplasma pneumoniae since the patient was receiving azithromycin prophylactically, but these assays should be performed if available because these are common community-acquired pathogens.
Transbronchial biopsy obtained 5 samples for histopathologic analysis. These contained lung parenchymal tissue and, in at least 3 samples, lung wall tissue. Histopathologic analysis found no evidence of acute rejection such as perivascular or interstitial mononuclear cell infiltrates.
Our patient stayed in the hospital a total of 4 days. Before her discharge, results from the viral panel from bronchoalveolar lavage were returned and were positive for HMPV. We therefore diagnosed acute or infectious bronchitis.
HUMAN METAPNEUMOVIRUS, AN EMERGING PATHOGEN
HMPV is an emerging pathogen responsible for respiratory tract infections, which are a significant cause of morbidity and death in immunocompromised patients.15 HMPV infection can present with clinical symptoms as benign as a self-limiting cough and rhinorrhea, or as severe as respiratory failure and death.
Nucleic acid amplification testing of respiratory secretions with reverse transcriptase polymerase chain reaction is the gold standard for HMPV diagnosis. A higher level of suspicion is warranted for secondary bacterial pneumonia, which is associated with increased mortality in immunocompromised patients.16
No guidelines or licensed antivirals currently exist, so treatment primarily consists of supportive care. Although ribavirin and intravenous immunoglobulin have been used in individual patients and series, randomized clinical trials have yet to be published.16,17 Unfortunately, infection does not provide long-term immunity,15 no antivirals have been licensed for prevention, and vaccine development is still in preclinical stages.16,17
FURTHER MANAGEMENT
3. Given our patient’s diagnosis of HMPV bronchitis, which of the following is the best next step in her management?
Transition to oral antibiotics
Decrease her immunosuppression
Repeat chest imaging
Supportive care only
Transitioning to oral antibiotics is appropriate in our immunocompromised patient, even though a viral pathogen has been identified, in view of her risk of developing secondary bacterial pneumonia. No high-quality evidence is available yet to guide the duration of treatment in immunocompromised patients, but our patient was discharged on a 3-day course of azithromycin at a higher dose to treat her acute infection, followed by continuation of a lower dose to prevent bronchiolitis obliterans syndrome. She was also instructed to continue using her home spirometer to monitor and ensure continuous improvement.
In an otherwise healthy patient, antibiotic treatment of acute bronchitis, which is most often of viral etiology,18 is not recommended, as outlined in clinical guidelines from the American College of Physicians18 and the Centers for Disease Control and Prevention.19
Procalcitonin, a serum marker of bacterial infections, has received interest as a tool to help decide whether to start or stop antibiotics. A Cochrane review found that measuring procalcitonin resulted in a lower risk of death and a shorter duration of antibiotic use, resulting in a lower risk of antibiotic-associated side effects.20 However, evidence is scarce regarding its utility in immunocompromised patients.21 Our patient’s procalcitonin level was low and thus could not be used to support the use of antibiotics.
Decreasing immunosuppression may be a useful adjunct to antimicrobial therapy, but this benefit may be outweighed by the risks of graft rejection and increased inflammation due to immune reconstitution syndromes.12 Current guidelines do not provide clear direction, and the decision to decrease immunosuppression should be made on a case-by-case basis by the transplant team.
Repeat chest imaging should be done based on clinical judgment. Because our patient was immunosuppressed and had developed a productive cough while in the hospital, a repeat chest radiograph was obtained before discharge. It showed that the focal opacity in her right lung had gotten smaller (Figure 2).
Chest radiograph on day 4 showing interval improvement in opacity superior to the right hilum (circle).
Supportive care only is recommended for an otherwise healthy patient with acute bronchitis. But in an immunocompromised patient, close follow-up with repeat blood testing and imaging and consideration of antibiotic therapy are important. Of note, high-dose corticosteroids are a risk factor for progression of respiratory tract infection in immunocompromised patients with HMPV infection,17 so their therapeutic use is not recommended. Antileukotrienes and antihistamines may also in theory be therapeutic based on their anti-inflammatory properties, but this has not yet been demonstrated clinically.22
Posthospitalization follow-up
Routine follow-up is important in the care of transplant patients, particularly after recent hospitalization.
Less than 1 week after going home, our patient had a complete blood cell count with differential, basic metabolic panel, serum phosphorus and magnesium, serum cytomegalovirus viral load, and serum human lymphocyte antigen antibody screen: all were negative or within normal limits. Pulmonary function testing was also performed, and the results were deemed stable overall compared with earlier results. At an outpatient clinic visit with the lung transplant team, she was found to have completely recovered.
TAKE-HOME POINTS
The differential diagnosis must be broad in transplant recipients when they present with even minor symptoms.
Although a self-limiting illness is possible, a high level of clinical suspicion is warranted in immunosuppressed patients such as transplant recipients, who are at great risk of rapid deterioration.
Early and comprehensive evaluation of transplant recipients is essential to determine appropriate treatment, as the choice of intervention may vary widely based on the diagnostic workup.
Serial radiographic imaging may not be appropriate unless new symptoms arise or symptoms worsen.
Community-acquired viral respiratory tract infections are common and can cause severe illness and death in solid-organ transplant recipients.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.