ABSTRACT
New-onset post-cardiac surgery atrial fibrillation (PCSAF) is a frequent complication with estimated incidence of 17% to 64%, depending on type of surgery. It is associated with higher mortality, morbidity, and predisposition to stroke and systemic embolism postoperatively. Standard care involves rate or rhythm control, in addition to antithrombotic therapy in those with history of stroke, transient ischemic attack, or high risk of systemic thromboembolism. However, risk of bleeding is not negligible, and treating physicians should weigh the risks and benefits before committing to postoperative anticoagulation therapy. More investigations are warranted to explore antithrombotic therapy benefit, particularly postoperative anticoagulation, considering the potentially self-limited nature of the arrhythmia and high risk of postoperative bleeding.
Anticoagulation should be weighed against potential risk of postoperative bleeding for patients with new-onset PCSAF as it is potentially transient and self-limited.
Avoid anticoagulation therapy for transient atrial fibrillation < 48 hours.
Anticoagulation is usually recommended for 4 to 6 weeks after atrial fibrillation conversion owing to enhanced risk of thrombosis resulting from persistent weak atrial contraction.
There is no exact consensus regarding the definition of new-onset post-cardiac surgery atrial fibrillation (PCSAF), and thus criteria for diagnosis are set by individual institutions. Our definition of new-onset atrial fibrillation is any duration detected on 12-lead electrocardiography or on a telemetry strip, developed in patients after cardiac surgery without previous diagnosis of atrial fibrillation, regardless of need for treatment. Cardiac surgery is defined as coronary artery bypass grafting (CABG), valve surgery, or a combination of both procedures. Studies have determined the postoperative timing of new-onset atrial fibrillation to range from the first 10 days postoperatively1 to the first 30 days after surgery,2 as well as during hospitalization only.3
The purpose of this review is to evaluate the studies, professional society recommendations, and expert thoughts on knowledge gaps relevant to anticoagulation therapy of new-onset PCSAF.
EPIDEMIOLOGY
With little change over the past 20 years, new-onset PCSAF is estimated to occur in the postoperative period in 17% to 40% after isolated CABG surgery,4–7 38% to 64% after heart valve surgery,3,4,7 and as high as 62% after combined procedures.1,3,4,6
New-onset PCSAF tends to develop within the first few days postoperatively,4,5 with the highest incidence seen on postoperative day 2, while most recurrence happens by postoperative day 3.5 Few patients develop new-onset atrial fibrillation in the very early postoperative period or 4 days or more after surgery.4
In patients who have no prior history of atrial fibrillation, new-onset PCSAF is usually transient in nature, as up to 80% revert to sinus rhythm within 24 hours,8 and the majority of patients (90%) are in sinus rhythm 6 to 8 weeks after discharge.4,9
THE IMPACT OF NEW-ONSET POST-CARDIAC SURGERY ATRIAL FIBRILLATION
Potential adverse events following new-onset PCSAF include thromboembolic complications, worsening of comorbid medical conditions, and increased mortality. Of interest, is the potentially increased rate of postoperative thromboembolic complications including stroke leading to the potential role of anticoagulation therapy in their prevention.10
Short-term complications
In multiple series, new-onset PCSAF had been associated with increased in-hospital postoperative stroke and increased mortality.3,6,11 These findings were similar in both on-pump (with cardiopulmonary bypass)3 and off-pump (without cardiopulmonary bypass) CABG surgery, and with and without valve surgery.11
On the other hand, other single-center studies failed to find an association between new-onset PCSAF and increased risk of in-hospital stroke.12,13 In one study, more than half of strokes occurred postoperatively rather than intraoperatively, and new-onset PCSAF was not associated with increased risk of stroke.13 However, older age and variables indicative of arteriosclerotic burden were risk factors for both intraoperative and postoperative stroke.13
Long-term complications
In most reports, new-onset PCSAF has been associated with worse long-term survival and increased thromboembolic complications.6,14–16 For instance, in the Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization trial of patients with left main coronary artery disease who underwent revascularization with either CABG or percutaneous coronary intervention, new-onset atrial fibrillation was an independent stroke predictor at three years in CABG patients.15
In another cohort study of patients who had undergone CABG surgery, risk of thromboembolism was lower in the PCSAF group than in the group with atrial fibrillation that was not valvular.14 Anticoagulation was associated with a lower risk of thromboembolic events in both groups, and thromboembolism was not significantly higher in patients with new-onset PCSAF compared with those who did not develop new-onset atrial fibrillation after surgery.
Risk of recurrence
There is considerable debate as to whether atrial fibrillation developing after cardiac surgery has a high risk of recurrence and thus whether extended anti-coagulation therapy to prevent thromboembolism can be beneficial. A recent meta-analysis analyzed 8 studies with a total of 1,157 participants monitored for new-onset PCSAF recurrence after discharge, all of whom were discharged in sinus rhythm and subsequently monitored by both noninvasive and invasive devices.17 Monitoring identified recurrence in 28.3 per 100 persons screened in the first 2 to 4 weeks after discharge using noninvasive techniques. Implanted devices identified recurrence in 61% to 100% of cases, suggesting that in-hospital PCSAF episodes are not transient. Most recurrences were asymptomatic and thus likely to be overlooked without the use of monitoring after hospital discharge.17
ANTICOAGULATION THERAPY FOR POST-CARDIAC SURGERY ATRIAL FIBRILLATION
Evidence behind anticoagulation and benefits
In medical patients with chronic or recurrent atrial fibrillation, the cause-and-effect relationship between atrial arrhythmias and thromboembolic events has been thoroughly examined18–21; however, this relationship has not been evaluated in the post-cardiac surgery setting. Current recommendations from the American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) 2014 and 2019 updated guidelines is to use warfarin (to target an international normalized ratio [INR] 2–3) and novel oral anticoagulants in patients with history of stroke, transient ischemic attack, or CHA2DS2-VASc score ≥ 2, defined as patients with congestive heart failure, hypertension, age ≥ 75, diabetes, stroke, vascular disease, age 65 to 74, and female.22,23
The question remains whether these guidelines are applicable to new-onset PCSAF in the postoperative period, taking into account the following facts:
Risk of bleeding may be significant in the postoperative period
CHA2DS2-VASc is a risk stratification tool that assesses annual stroke risk, however, has not been studied as an instrument for predicting postoperative stroke and embolic phenomena
In current literature, high-quality evidence of early postoperative antithrombotic therapy benefits in thromboembolic complications is lacking.
Kollar and colleagues24 described a retrospective study regarding benefits of anticoagulation in the postoperative period of 2,960 patients after CABG with 32 patients having had a postoperative stroke. The study was unique in that it examined the temporal relationship between postoperative atrial fibrillation and stroke. Seventeen of these patients continued to maintain sinus rhythm during their hospitalization. Of the remaining 15 patients, 9 had neurologic deficits before the first episode of atrial fibrillation. Of the 6 patients with atrial fibrillation preceding neurologic events, three strokes occurred within 1 week after spontaneous conversion to normal sinus rhythm. One patient with preoperative as well as intraoperative atrial fibrillation underwent emergency CABG surgery and woke up with a stroke. In the remaining two cases, the atrial fibrillation or atrial flutter episodes lasted less than 6 hours each before the onset of neurologic events. Authors concluded that aggressive anticoagulation as suggested in current guidelines could not have decreased the already low incidence of postoperative stroke.24 The study, however, was underpowered making it difficult to draw a conclusion that is generalizable to a large cohort. An adequately powered prospective registry evaluating the benefits of anticoagulation in the early postoperative period is warranted.
Risk of postoperative bleeding with anticoagulation
The questionable rationale of early anticoagulation in reducing postoperative stroke is impacted by the risk of associated bleeding in the postoperative period. For instance, there was a significant increase in large pericardial effusions and tamponade occurring one week or more after surgery in a study of patients treated with warfarin compared with the antiplatelet control group, especially when excessively anticoagulated (INR above therapeutic target).25
Bleeding other than at the surgical site might be relevant when determining use of anticoagulants, particularly with gastrointestinal hemorrhage. In a single-center, retrospective analysis of 9,017 patients undergoing cardiac surgery, the incidence of endoscopy-requiring gastrointestinal hemorrhage was 1.01%, and 30-day mortality was higher in patients who bled compared with patients who did not.26 The study was limited in that only bleeding events requiring intervention were included.
HAS-BLED is a risk score, defined as hypertension, abnormal renal or liver function, stroke, bleeding history or predisposition, labile INR, elderly (age > 65), and drugs or alcohol concomitantly, that is often used to determine use of anticoagulation therapy by evaluating risks and benefits in the general population with atrial fibrillation that is not valvular. However, validity in patients after surgery has not been evaluated.27 Thus, a high-quality risk stratification tool to identify bleeding in surgical patients would be of great clinical value.
Recommendations of professional societies
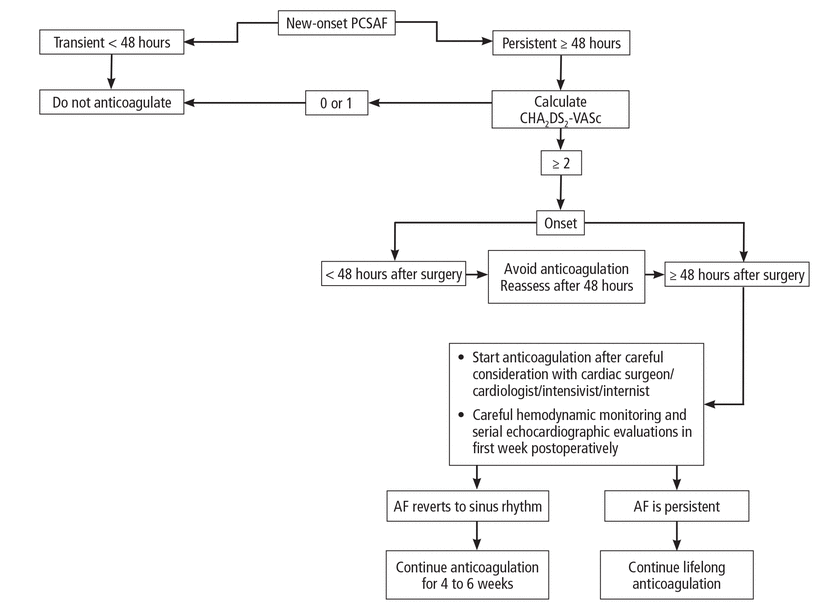
The AHA/ACC/HRS guidelines recommend considering using anticoagulation medication for patients who develop new-onset postoperative atrial fibrillation as administered for nonsurgical patients.22 The American College of Chest Physicians suggests weighing the AHA/ACC/HRS recommendations in the context of the usually transient and self-limited duration of new-onset PCSAF against the potential risk of bleeding and recommends starting anticoagulation therapy if atrial fibrillation persists 48 hours and beyond. They further state that therapy should be continued for 30 days after the return to normal sinus rhythm because of persistent atrial contraction impairment and possible enhanced risk of thrombosis following cardioversion to sinus rhythm.28 The Canadian Cardiovascular Society Atrial Fibrillation Guidelines recommend continuing anticoagulation for new-onset PCSAF for a minimum of 6 weeks,29 although the American College of Chest Physicians and AHA/ACC/HRS did not provide details regarding timing of initiation of antithrombotic therapy. A report from the European Association for Cardio-Thoracic Surgery does not recommend immediate full antithrombotic therapy when new-onset atrial fibrillation develops within 48 hours of cardiac surgery, due to increased risk of cardiac tamponade.30 Table 1 provides a summary of these recommendations,22,23,28–30 and Figure 1 shows our proposed algorithm for management of new-onset PCSAF.
Recommendations of professional societies
Proposed algorithm for management of new-onset atrial fibrillation following cardiac surgery.
AF = atrial fibrillation; CHA2DS2-VASc = congestive heart failure, hypertension, age ≥ 75, diabetes, stroke, vascular disease, age 65 to 74, and female; PCSAF = post-cardiac surgery atrial fibrillation
Selection of drugs
The American College of Chest Physicians and AHA/ACC/HRS recommend anticoagulation therapy with warfarin, with or without heparin for atrial fibrillation.22,28 Novel oral anticoagulants have been included in the 2014 and 2019 updated AHA/ACC/HRS guidelines.22,23 More recently, the 2019 report added idarucizumab and andexanet alfa for the reversal of direct thrombin and activated factor Xa inhibitors, respectively.23
In one retrospective review, patients were administered anticoagulation with either warfarin or novel oral anticoagulants for new-onset PCSAF in the postoperative period; both drug classes were found to be safe and effective methods of anticoagulation with no significant difference between outcomes of strokes, postoperative hemorrhage, and hospital length of stay.31 The study was small and limited to the hospital postoperative period. Two other small pilot studies compared edoxaban32 and apixaban33 to warfarin for new-onset PCSAF and demonstrated safety and efficacy while suggesting larger scale clinical trials for further evaluation. A randomized clinical trial comparing rivaroxaban and warfarin for new-onset PCSAF is currently under way.34
Monitoring of therapy and discontinuation of anticoagulants
There is a lack of evidence regarding monitoring following discharge for new-onset PCSAF patients who revert to sinus rhythm before discharge. Consequently, some hospitals have developed institutional policies regarding follow up and monitoring of patients that relies on expert opinion. For instance, a feasibility cross-sectional study with a unique idea of self-monitoring assessed 42 patients who had undergone cardiac surgery (CABG, valve surgery, or combination) for new-onset PCSAF recurrence using smart phone handheld electrocardiogram devices.35 Study participants were instructed to record rhythm for 30 seconds, 4 times daily, for 4 weeks after discharge. Owing to the feasibility of the study, there was no control group, randomization, or blinding. Self-monitoring identified 24% with atrial fibrillation recurrence within 17 days of hospital discharge. Surprisingly, patients with atrial fibrillation recurrence were younger and had a lower CHA2DS2-VASc score than those without atrial fibrillation recurrence. Results should be interpreted with caution owing to the small sample size, infrequent self-monitoring, and study duration. Additional atrial fibrillation episodes may have been detected with a larger sample size and longer daily monitoring.
CONCLUSIONS
Adequately powered, large prospective studies are needed to investigate the relationship between transient atrial fibrillation and subsequent thromboembolic events in the postoperative period. Assessment of risk of hemorrhage in the immediate (first 48 hours) and later (≥ 48 hours to 7 days) postoperative period must be validated for cardiac surgery patients in large scale settings and may help to predict bleeding risk and allow for evaluation of postoperative anticoagulants. It is important to identify risk assessment models other than CHA2DS2-VASc score while considering operative technique to help identify patients at high risk in the setting of cardiac surgery. Finally, multicenter randomized trials investigating the benefits and risks of anticoagulation therapy indicated for transient atrial fibrillation early after cardiac surgery are warranted.
DISCLOSURES
The authors report no relevant financial relationships, which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.