A 34-year-old male with a history of syphilis and with human immunodeficiency virus on a home regimen of dolutegravir and the combination of darunavir, cobicistat, emtricitabine, and tenofovir alafenamide presented to the emergency department with persistent rectal pain, yellow rectal discharge, widespread skin lesions, and episodes of fever, with a maximum temperature of 102.9°F (39.4°C). The rectal pain and discharge had started 1 week earlier, and 4 days after that, he developed skin lesions on the face that quickly spread to the rest of his body.
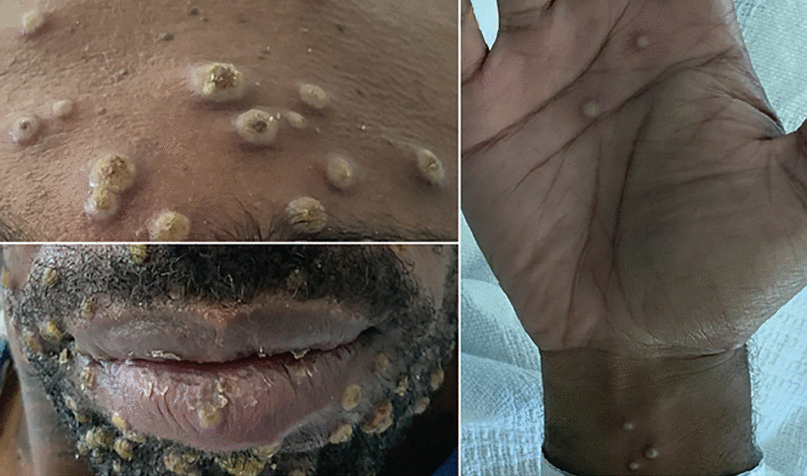
Examination of the skin revealed diffuse pustular lesions involving the face, chest, back, all 4 extremities, genitalia, and palms (Figure 1), and the patient was admitted to the hospital for further evaluation.
The patient presented with widespread diffuse pustular lesions, including the face and palms, diagnosed as mpox.
Examination of the anal region revealed multiple unroofed papules with serous drainage at the anal sphincter. Anal lesions were swabbed and sent for testing for mpox virus and herpes simplex virus. Results of laboratory testing revealed a white blood cell count of 13.1 × 109/L (reference range 3.5–10.5), a human immunodeficiency viral load of 22,700 copies/mL, and a CD4 count of 447 cells/mm3 (500–1,200). Results of a quantitative rapid plasma reagin test were consistent with treated past syphilis infection.
Computed tomography suggested a perirectal abscess, with mucosal hyperenhancement around the rectum, mild circumferential perirectal edema, and outpouching along the left lateral rectal wall of less than 1 cm.
Owing to high suspicion for mpox (formerly monkeypox) virus infection, the patient was placed on isolation precautions and was started on tecovirimat 600 mg twice daily for 14 days, and doxycycline for proctitis. The patient’s febrile episodes stopped on day 2 of hospitalization. Marked improvement in the anal discharge was noted on day 4, though the rectal pain persisted with bowel movements. The skin lesions improved, developing a hard crust and exhibiting decreased drainage. Days later, on hospital day 7, the lesion swab resulted positive for mpox virus.
MPOX EPIDEMIOLOGY
As of February 1, 2023, the US Centers for Disease Control and Prevention reported more than 88,000 confirmed cases of mpox globally in more than 110 locations, over 90% of which have not historically reported mpox infections.1 Nearly 31,000 cases have been confirmed across the United States, including pediatric cases, and 33 fatalities were reported, the majority in severely immunocompromised adults.1,2
Despite being first witnessed in captive cynomolgus monkeys, rodents and small forest mammals have been noted to be the attributed source of zoonotic transmission, with the first human case of mpox reported in 1970 in a 9-month-old child in the Democratic Republic of the Congo.3,4 In the United States, human cases of mpox have historically been described in laboratory workers, pet shop workers, and veterinarians after direct contact with an infected animal.
The exact mode of transmission is still under investigation, although it seems that human-to-human transmission is primarily due to contact with lesions, infected bodily fluids, or large respiratory droplets.3,4 Contact with recently contaminated objects or surfaces used by an infected individual is also considered a risk factor for transmission.3 With respect to the current (ie, 2022) outbreak, mpox cases have been concentrated in men who have sex with men.3,4
CLINICAL PRESENTATION OF MPOX
The clinical presentation of mpox often begins with a nonspecific prodromal period consisting of 1 to 5 days of fever, sweats, chills, headache, back pain, myalgia, and lymphadenopathy.4 Within 1 to 5 days from fever onset, a rash appears first as macules, followed by papules, then vesicles, and finally pea-sized hard pustules. These pustules become umbilicated, develop crust, and eventually desquamate, leading to resolution of the rash in 7 to 14 days.4
However, in the current outbreak, patients may present with a less severe prodrome and increased prevalence of vesicular lesions in the genital and perineal regions. In addition, symptoms may include anorectal pain or pharyngitis. The differential diagnosis of pustular lesions consists of several infectious processes including mpox, herpes simplex virus, molluscum contagiosum, cutaneous cryptococcosis, cutaneous cytomegalovirus, syphilis, and lymphogranuloma venereum.4,5
MANAGEMENT OF MPOX
Many patients with mpox will recover within 2 to 4 weeks without any medical intervention.3,4 Severe cases can occur, more commonly in children and immunocompromised individuals, with a case-fatality rate of 1% to 11%.4 Tecovirimat is approved by the US Food and Drug Administration for the treatment of smallpox and may be considered for patients with or at increased risk of severe mpox through the Expanded Access Investigational New Drug Protocol for treatment of nonvariola orthopoxviruses like mpox during an outbreak.4 Vaccinia immune globulin intravenous, brincidofovir, and cidofovir are currently being evaluated.4 Mpox vaccination should be offered to individuals at high risk of exposure or after known or presumed exposure to mpox virus.1
PATIENT OUTCOME
The patient was discharged on hospital day 8 with continuation of his home antiretroviral medication, a 4-day course of oxycodone for pain management, and instructions to isolate from human contact for 4 to 6 weeks, until lesions had disappeared, and new skin had formed underneath all scabs.
His most recent follow-up with an outside dermatologist at 6 months after discharge revealed resolution of mpox lesions and postinflammatory hyperpigmentation of the involved sites.
TAKE-HOME POINTS
Mpox should be included in the differential diagnosis when assessing patients with new papulovesicular or vesiculopustular lesions. In contrast to previous outbreaks, the current outbreak is primarily driven by human transmission, may lack the characteristic prodrome or lymphadenopathy, and may present with anorectal pain or pharyngitis. While most cases are self-limited, tecovirimat may be considered in patients with severe disease.
DISCLOSURES
Dr. Andrasik has disclosed consulting for Bristol-Myers Squibb. Dr. Fernandez has disclosed consulting for Abbvie Pharmaceuticals, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Mallinckrodt, Novartis, and UCB; teaching and speaking for Abbvie Pharmaceuticals, Kyowa Kirin, Mallinckrodt, and Novartis; work as advisor or review panel participant for Abbvie Pharmaceuticals; research or principal investigator for Alexion; and awarded a grant from Pfizer to fund a medical dermatology fellowship position. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.