A 53-year-old woman presented to the emergency department with 6 months of progressively worsening episodic upper abdominal pain. The pain was sharp and radiating to the back and exacerbated by eating, which caused her to avoid oral intake and led to a more than 12-kg (26.5-lb) weight loss. She also described intermittent nausea and fatigue but had no fevers, yellowing of the eyes or skin, pruritis, swelling, vomiting, diarrhea, constipation, or bloody stools. She had no history of tobacco, alcohol, or illicit drug use and had no significant travel history.
In her medical history, the patient reported recurrent epistaxis that started in adolescence, followed by the emergence of telangiectasis involving her lips, tongue, fingers, and feet during her 20s. Her epistaxis required intermittent intravenous iron infusions, but she denied a history of gastrointestinal bleeding, stroke, seizures, shortness of breath, or peripheral swelling. She had no history of migraines. Family history included hypertension and epistaxis in her mother, hypertension in her father, and epistaxis in her only sibling. Her only medication was oxymetazoline.
On examination, the patient was afebrile with a blood pressure of 109/54 mm Hg, heart rate 72 beats per minute, and oxygen saturation 98% on room air. She did not appear to be in acute distress. Extraocular movements were intact with no scleral icterus. Cardiovascular examination showed a regular rate and rhythm with no detectable murmur, rub, or gallop on auscultation. There was no jugular venous distention, parasternal heave, or peripheral edema. Breathing was unlabored and symmetric with breath sounds clear to auscultation bilaterally. The abdomen was soft, not distended, and mildly tender to palpation in the right upper quadrant, with no rebound or involuntary guarding. A negative Murphy sign was noted. There was a nontender epigastric pulsatile mass on palpation, and an abdominal bruit was identified on auscultation. Multiple pinpoint telangiectasias were present on the lips, tongue, and fingers. There were no telangiectasias of the nail beds. No jaundice of the skin was noted. Neurologic examination demonstrated intact motor and sensory function with no focal deficits.
Laboratory tests showed iron deficiency anemia (Table 1). Renal function tests results were within normal limts, as were alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, bilirubin, troponin I, and lipase. The coagulation profile was normal. No recent test results were available for comparison.
The patient’s laboratory test results during initial presentation at the emergency department
Electrocardiography demonstrated normal sinus rhythm with no prolonged intervals and no evidence of left or right atrial enlargement. Right-axis deviation (negative lead I) was present. There was no dominant R wave in lead V1 or dominant S wave in leads V5 and V6. There was normal R-wave progression, and ST-T wave abnormalities were absent.
DIFFERENTIAL DIAGNOSIS AND CHOICE OF IMAGING STUDY
1. Which imaging test is the most appropriate to obtain next?
Computed tomography of the abdomen
Magnetic resonance cholangiopancreatography
Abdominal Doppler ultrasonography
Plain radiography of the abdomen
The differential diagnosis of acute-on-chronic upper abdominal pain is broad. It includes the following:
Hepatobiliary pathology (eg, symptomatic cholelithiasis, acute cholecystitis, cholangitis, hepatitis)
Visceral organ inflammation (eg, pancreatitis, appendicitis, diverticulitis)
Autoimmune or inflammatory conditions (eg, celiac disease, inflammatory bowel disease)
Vascular disorders (eg, abdominal aortic aneurysm, mesenteric ischemia)
Malignancy
Obstruction
Infection
Genitourinary disorders (eg, nephrolithiasis)
Gynecologic disorders
Cardiac disease (eg, acute coronary syndrome)
Functional gastrointestinal disorders.
The findings did not yet support a particular diagnosis. Her iron deficiency anemia was likely secondary to her recurrent epistaxis, which at this point had an unclear association with her acute presentation.
The presence of a palpable pulsatile abdominal mass with a bruit on auscultation initially raised suspicion for an abdominal aortic aneurysm, which would have been unusual in a woman in her 50s with no history of tobacco use or other atherosclerotic risk factors. Diagnostic mimics of abdominal aortic aneurysm include malignancy, pancreatic pseudocyst, and enlargement of the liver (particularly in cases with prominent vascularity). Otherwise, a largely benign physical examination with a nonacute abdomen and laboratory results with no readily apparent hepatobiliary injury pattern minimized concern for acute ductal impaction, visceral organ inflammation, or infection.
The appropriate initial imaging modality in this patient was abdominal Doppler ultrasonography, as it can quickly and accurately assess for the most common pathologic considerations in acute right upper quadrant pain and for abdominal aortic aneurysm.1,2 Ultrasonography findings can often generate an actionable diagnosis. This supports its initial use over computed tomography which, although able to offer more comprehensive morphological characterization in certain disease states, is more costly and exposes the patient to ionizing radiation. Plain radiography of the abdomen would be most appropriate to evaluate for pneumoperitoneum, which was of lower concern in this patient with no predisposing factors for hollow organ perforation and no evidence of acute abdomen on physical examination. Magnetic resonance cholangiopancreatography can provide detailed information about the pancreaticobiliary ductal system, but it takes more time, is more technically difficult, and would have been premature in the initial diagnostic evaluation.
CASE CONTINUED: IMAGING RESULTS
Abdominal aortic ultrasonography revealed no aneurysm, with a maximum aortic diameter of 2.2 cm. Ultrasonography of the right upper quadrant showed no thickening of the gallbladder or gallstones, no pericholecystic fluid, and a nondilated common bile duct; Murphy sign was negative. The liver demonstrated heterogeneous echogenicity with a prominent left lobe and hepatic vascularity, including enlarged hepatic arteries and veins.
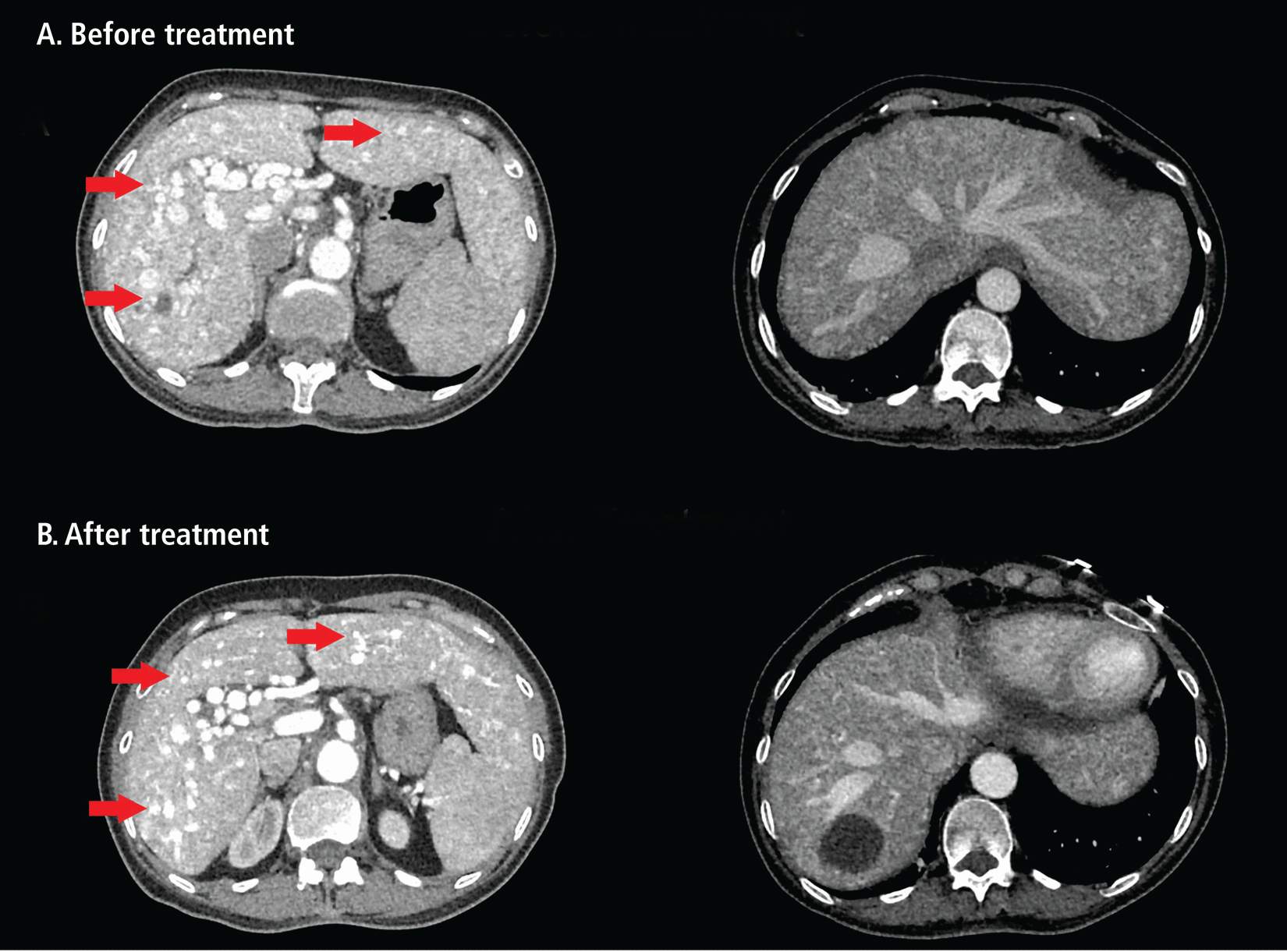
These ultrasonography findings prompted follow-up computed tomography 3-phase imaging of the abdomen and pelvis, which showed extensive arteriovenous malformations (AVMs) predominantly in the liver involving shunts from the right and left hepatic arterial branches to the hepatic portal vein (Figure 1A). No dissection or aneurysm of the aorta as it traversed the thorax and abdomen was noted. There was no intrahepatic ductal enlargement, and no observable extrahepatic biliary or visceral organ abnormalities were noted.
Computed tomography 3-phase imaging of the abdomen and pelvis showed (A) extensive arteriovenous malformations in the liver (red arrows), predominantly involving shunts from the right and left hepatic arterial branches to the hepatic portal vein. (B) Repeat computed tomography imaging of the abdomen and pelvis after 3 months of treatment with bevacizumab revealed an unchanged appearance of the liver, with extensive arteriovenous malformations (red arrows).
Computed tomography angiography of the chest showed cardiomegaly, an enlarged main pulmonary artery, and small AVMs in the bilateral lower lobes.
With no perceivable biliary compromise, the patient was treated with a proton pump inhibitor and oral analgesics, which abated her pain, and was discharged with the recommendation to follow up with her primary care physician.
DIAGNOSIS
2. What is the most likely underlying cause of this patient’s epistaxis, telangiectasias, and solid-organ AVMs?
Limited systemic sclerosis
Ataxia-telangiectasia
Generalized essential telangiectasia
Hereditary hemorrhagic telangiectasia (HHT)
HHT is a rare autosomal dominant hereditary vascular disorder that leads to mucocutaneous telangiectasias and visceral AVMs.3,4 The most well-characterized sequelae include recurrent epistaxis and solid organ bleeds. Clinical diagnosis of HHT in adults is made via the Curaçao criteria,5 which require the presence of the following:
Recurrent spontaneous epistaxis
A first-degree relative with HHT
Multiple mucocutaneous telangiectasias
Visceral AVMs.
Fulfillment of 3 or more criteria yields a definite diagnosis.
Most patients with HHT have pathogenic variants of the ENG, ACVRL1, and SMAD4 genes, which encode proteins of the transforming growth factor-beta superfamily (endoglin, activin A receptor-like type 1, and SMAD4, respectively).6 Insults to this pathway result in dysregulated angiogenesis and vascular remodeling, leading to the dilated and weakened vessels that comprise telangiectasias and AVMs. Patients with pathogenic ENG variants have been reported to be more likely to develop pulmonary and cerebral AVMs, while those with ACVRL1 variants have more often demonstrated hepatic involvement as well as heritable pulmonary arterial hypertension.7,8 However, there is now less emphasis on these genotype-phenotype associations as there can be significant overlap of symptoms and organ involvement across the HHT genotypes.8
The presence of multiple visceral AVMs has no established pathophysiologic relationship with limited systemic sclerosis, ataxia-telangiectasia, or generalized essential telangiectasia. Limited systemic sclerosis is an autoimmune disease that may lead to telangiectasias and epistaxis; however, additional manifestations include calcinosis, Raynaud phenomenon, esophageal dysfunction, and sclerodactyly.9 Ataxia-telangiectasia syndrome is a rare autosomal recessive neurodegenerative disease caused by a defect in the ATM gene, resulting in visible telangiectasias, impaired movement secondary to cerebellar defects, and variable immunodeficiencies.10 Generalized essential telangiectasia is a rare benign condition, currently of unknown etiology, characterized by progressive onset of diffuse, symmetrical telangiectasias with no other systemic or extracutaneous manifestations.11
CASE CONTINUED: EVALUATION AT HHT CLINIC
One month following discharge from the emergency department, the patient presented to our HHT clinic. On evaluation, she reported a history of recurrent epistaxis and diffuse telangiectasias in her mother. Her epistaxis was described as debilitating (epistaxis severity score of 7.1), with episodes lasting longer than 15 minutes and occurring multiple times per day.12 A colonoscopy obtained 2 months before presentation did not show gastrointestinal AVMs or polyps. She continued to experience throbbing right upper quadrant pain leading to reduced oral intake and ongoing weight loss.
Abdominal examination showed a right upper quadrant thrill and flow murmur. Laboratory results revealed worsening iron deficiency anemia. The ammonia level was 82 μmol/L (reference range ≤ 72 μmol/L) and B-type natriuretic peptide was 113 pg/mL (≤ 100 pg/mL). Electrolytes, renal function tests, and coagulation profile were normal.
A transthoracic echocardiogram with agitated saline contrast showed a left ventricle with mild hypertrophy and ejection fraction of 60% to 65%, a right ventricle with mild dilation and tricuspid annular plane systolic excursion of 32 mm (≥ 17 mm), and delayed appearance of bubbles in the left cardiac chambers suggestive of a grade 2 intrapulmonary shunt.
Right heart catheterization showed the following:
Mean pulmonary artery pressure 28 mm Hg (≤ 20)
Pulmonary arterial wedge pressure 10 mm Hg (≤ 15)
Pulmonary vascular resistance 1.5 Wood units (≤ 2)
Cardiac output 12.1 L/minute (5–6)
Cardiac index 7.7 L/minute/m2 (2.5–4).
There was a step-up in oxygen saturation indicating a left-to-right shunt at the level of the liver consistent with hepatic AVMs. Ventilation-perfusion lung scan did not identify areas of mismatched perfusion defects.
PULMONARY HYPERTENSION IN HHT
3. What is the interpretation of the patient’s right heart catheterization findings?
Precapillary pulmonary hypertension
Postcapillary pulmonary hypertension
Combined pre- and postcapillary pulmonary hypertension
Pulmonary hypertension with a high cardiac output state
A mean pulmonary artery pressure greater than 20 mm Hg on right heart catheterization indicates the presence of pulmonary hypertension, which can be divided into precapillary, postcapillary, and unclassified causes.13 Precapillary pulmonary hypertension is characterized by increased pulmonary vascular resistance due to pathologic remodeling and is defined as a pulmonary arterial wedge pressure of less than or equal to 15 mm Hg and pulmonary vascular resistance greater than 2 Wood units. Common causes include pulmonary arterial hypertension (eg, inherited, drug-induced, idiopathic) and chronic lung disease or hypoxia.
Pulmonary hypertension in the setting of chronic and recurrent pulmonary thromboembolism, termed chronic pulmonary thromboembolic hypertension, presents with precapillary pulmonary hypertension on hemodynamics; however, this etiology would be associated with areas of mismatched perfusion defects on ventilation-perfusion lung scan.14
Postcapillary pulmonary hypertension is caused by increased pulmonary venous pressure and is defined as a pulmonary arterial wedge pressure greater than 15 mm Hg and pulmonary vascular resistance of 2 Wood units or less. This is most often observed in the setting of left-sided heart failure.
Combined precapillary and postcapillary pulmonary hypertension demonstrates a pulmonary arterial wedge pressure greater than 15 mm Hg and pulmonary vascular resistance greater than 2 Wood units. It can be seen in a subset of cases of left-sided heart failure in which chronically elevated filling pressures promote precapillary pulmonary remodeling, and may be associated with worse clinical outcomes.15
Finally, unclassified pulmonary hypertension is defined by a pulmonary arterial wedge pressure less than or equal to 15 mm Hg and pulmonary vascular resistance of 2 Wood units or less. This can be evident in cases of elevated pulmonary blood flow, such as hyperthyroidism or a high cardiac output state.
Pathophysiology of AVMs
Although patients with HHT (especially those with ACVRL1 variants) are predisposed to heritable pulmonary hypertension, the presence of a high cardiac output state is a more common cause of pulmonary hypertension, as seen in this patient. High cardiac output in patients with HHT is a reflection of the inherent and essential pathophysiology of AVMs. Indeed, hepatic AVMs generate low-resistance vascular connections that may result in excess shunting and ischemia. Liver disease phenotypes associated with HHT include high cardiac output and portal hypertension as well as ischemic pathology like ischemic cholangiopathy and mesenteric steal syndrome. A high cardiac output state and ischemic cholangiopathy are the result of arteriovenous shunting, portal hypertension the result of arterioportal shunting, hepatic encephalopathy the result of portovenous shunting, and mesenteric artery steal syndrome the result of high-flow shunting from the right gastric artery, pancreaticoduodenal arteries, and gastroduodenal artery.16
In a high cardiac output state, AVMs create vascular connections that result in a high-flow state, leading to elevated pulmonary artery pressures. Chronically defective hepatic perfusion and excess neurohormonal activation can lead to progression from a high cardiac output state to high-output heart failure. This is evidenced by signs and symptoms of pulmonary and systemic congestion as well as a significant postcapillary component of pulmonary hypertension on hemodynamic assessment.17
Left-to-right shunting in hepatic AVMs may also result in bypassing of the peribiliary plexus, with subsequent bile duct ischemia. Chronic hypoperfusion eventually precipitates intrahepatic bile duct fibrosis with segmental dilation and strictures, resembling Caroli disease, as well as necrosis with leakage that may form a biloma.17 Patients will present with nonspecific abdominal symptoms of biliary colic. Biochemical testing, which often demonstrates a cholestatic injury pattern, often lags behind symptom onset and does not reflect the severity of ischemic injury. However, imaging in this patient did not demonstrate observable dilations or strictures of the biliary tree to support a diagnosis of ischemic cholangiopathy.
The presence of large AVMs has been described in cases of mesenteric ischemia. The AVMs result in a steal phenomenon characterized by progressive postprandial abdominal pain and avoidance of oral intake, and may even progress to ischemic colitis.18,19 In this patient, the sizable degree of shunting through hepatic AVMs and subsequent high-flow state also conceivably reduced perfusion of adjacent arterial beds (ie, right gastric artery, pancreaticoduodenal arteries, and gastroduodenal artery), leading to ischemic pathology and a mesenteric steal phenomenon causing chronic postprandial abdominal pain.
CASE CONTINUED: GENETIC TESTING AND TREATMENT
Genetic testing revealed a heterozygous pathogenic ACVRL1 c.935A>C (p.His312Pro) gene variant, consistent with the patient’s diagnosis of HHT. The patient received intravenous iron transfusions and was started on bevacizumab, with an induction regimen of 5 mg/kg every 2 weeks for 6 doses followed by an infusion every 4 months.
BEVACIZUMAB THERAPY
4. What is the mechanism of action of bevacizumab?
Inhibition of vascular endothelial growth factor A
Inhibition of fibroblast growth factor receptor
Inhibition of platelet-derived growth factor receptor A
Inhibition of epidermal growth factor receptor
Bevacizumab is an inhibitor of vascular endothelial growth factor A, which impedes neoangiogenesis and promotes regression of existing dysplastic vessels.20 Hence, its use in HHT-related liver disease may reduce the burden of arteriovenous shunting and improve hepatic perfusion. In patients with hepatic AVMs, high cardiac output refractory to salt and water restriction and diuretic therapy may be successfully treated with bevacizumab, with small controlled studies reporting improvement of symptoms and normalization of the cardiac index.21,22 Importantly, this clinical improvement has been shown to obviate the need for liver transplantation. A study of 3 patients with HHT and ischemic cholangiopathy described improvement in abdominal pain and functional status as well as resolution of cholestatic injury with the use of bevacizumab.23 Interestingly, patients also demonstrated radiographic evidence of clinical improvement (ie, reduction in burden of hepatic AVMs) at 1 year. Another report of 1 patient with ischemic cholangiopathy detailed a lack of clinical response to bevacizumab therapy and the onset of adverse thromboembolic events.24
Abdominal angina due to a mesenteric arterial steal phenomenon caused by AVMs of pancreaticoduodenal arteries in HHT has been described previously.16 The case presented here details the presentation of a mesenteric steal phenomenon along with other hepatic phenotypes in a patient with HHT, and also represents the novel finding of a positive response to bevacizumab therapy in this setting.
CASE CONCLUSION
The patient’s pulmonary AVMs were too small to coil and therefore were monitored, with no intervention. The patient underwent sclerotherapy of multiple nasal and oral telangiectasias by otorhinolaryngology. On follow-up evaluation after 3 months of bevacizumab therapy, improvement in hemoglobin levels was observed (Table 2). The patient reported significant reductions in epistaxis (epistaxis severity score 3.15) and abdominal pain, along with markedly improved tolerance for oral intake and an associated weight gain of 5 kg (11 lb). Additional ongoing therapy for the patient’s anemia included oral ferrous sulfate (325 mg every other day); oxymetazoline was used for supplementary control of epistaxis. Notably, repeat computed tomography imaging of the abdomen and pelvis showed an unchanged appearance of the liver with an extensive AVM burden (Figure 1B).
The patient’s laboratory test results at time of treatment and follow-up
Additional screening recommendations for patients with HHT include brain magnetic resonance imaging to evaluate for AVMs. Patients should also undergo a screening colonoscopy to evaluate for high-risk AVMs as well as for polyps, which have a specific association with a rare subtype of HHT caused by mutations in the SMAD4 gene, juvenile polyposis and HHT syndrome.25 These screening interventions did not show abnormalities in our patient.
HHT MANAGEMENT CONSIDERATIONS
This patient’s chronic pain profile and clinical presentation suggested significant arteriovenous shunting, which likely resulted in progressive hypoperfusion of the mesenteric vasculature and development of the mesenteric steal syndrome. After starting bevacizumab therapy, the patient reported considerable symptomatic improvement, while radiographic evaluation revealed an unchanged burden of AVMs in the liver. This may indicate that bevacizumab produced therapeutic benefit at the level of the microvasculature that was unobservable on computed tomography imaging. The shorter follow-up duration of 3 months must also be noted. Additionally, computed tomography angiography may not adequately assess changes in the degree of the mesenteric steal effect, as it is unable to capture pre- to postprandial vessel dilation.
Iron deficiency anemia is a common complication in HHT due to frequent bleeding events, primarily in the form of epistaxis and gastrointestinal hemorrhage.26 The resulting low blood viscosity can also exacerbate the high-flow state observed in hepatic AVMs, as well as paradoxically increase the risk for thrombotic events (eg, pulmonary embolism, cerebrovascular accidents). Treatment of epistaxis includes moisturizing topical agents, oral tranexamic acid, and ablative therapies.3 Refractory cases are considered for management with systemic antiangiogenic treatment or more intensive surgical intervention (eg, septodermoplasty, nasal closure).
Liver transplantation is indicated in cases of HHT-related liver disease refractory to medical management.3 This is especially relevant in such cases because alternative treatment modalities such as hepatic artery ligation or embolization are not advised because of high postprocedure mortality rates. Specifically, consideration for liver transplantation is recommended for patients with HHT who have high-output heart failure despite diuretic and antiangiogenic therapy, biliary ischemia, or severe complications of portal hypertension.3 Patients with HHT-related liver disease are eligible for model for end-stage liver disease exception points, which can help facilitate transplantation, and 10-year posttransplant survival rates may exceed 80%.27–29 Notably, this patient was being evaluated for liver transplantation in the setting of high cardiac output and a declining functional status. After receiving bevacizumab therapy, she had significant improvement in exercise capacity and abdominal pain. An associated weight gain effectively halted further consideration of transplantation at that time.
The presence of large pulmonary AVMs can cause significant hypoxemia, limiting patient functional status. Therefore, a multidisciplinary approach involving pulmonology, hematology, hepatogastroenterology, and otolaryngology is recommended to effectively manage this complex condition.
CONCLUSION
This patient’s case highlights the physiologic impact of AVMs in patients with HHT. Depending on the size and type of connection, a high burden of arteriovenous shunting can manifest in a high cardiac output state or portal hypertension, as well as abdominal ischemic phenotypes such as ischemic cholangiopathy or mesenteric steal syndrome. Hepatic AVMs in this patient led to multiple clinical syndromes, including a high cardiac output state from arteriovenous shunting, arterioportal shunting on imaging, hyperammonemia due to portovenous shunting, and mesenteric steal syndrome. The patient had a favorable response to bevacizumab therapy. Importantly, a clinical presentation with nonspecific signs and symptoms and no immediately notable biochemical findings, as seen in this patient, may lead clinicians to conclude their investigation prematurely.
TAKE-HOME POINTS
HHT is a rare inherited vascular disorder characterized by a positive family history, recurrent epistaxis, mucocutaneous telangiectasias, and visceral AVMs. Management involves a multidisciplinary team, ideally at an HHT Center of Excellence.
Pulmonary hypertension is exceedingly common in this population; 2 causes related to the pathophysiology of HHT are hepatic AVM–associated high cardiac output and heritable pulmonary arterial hypertension.
Pulmonary hypertension is categorized into precapillary, postcapillary, combined, and unclassified etiologies, depending on the pulmonary arterial wedge pressure and pulmonary vascular resistance.
The presence of a low-resistance, high-flow AVM in the liver can generate numerous phenotypes, including high cardiac output and a mesenteric steal phenomenon; treatment of these disease entities includes bevacizumab, an inhibitor of vascular endothelial growth factor A.
DISCLOSURES
Dr. Zumberg has disclosed teaching and speaking for the American Society of Hematology. Dr. Justice has disclosed teaching and speaking for Medtronic and consulting for Medtronic and 3D Matrix. Dr. Ataya has disclosed consulting for Savara and United Therapeutics Corporation. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.