A 60-year-old black man presented to our clinic with ischemic pain at rest in the right foot and dry gangrene of the forefoot and big toe (Figure 1).
At presentation, the patient had dry gangrene of the right hallux and an interdigital ulcer.
The patient had an extensive medical history that included the following:
Multivessel coronary artery disease, for which he had undergone coronary artery bypass grafting 1 year previously
Chronic limb-threatening ischemia in the left leg, for which he had undergone a left popliteal-to-dorsalis pedis artery bypass
Type 2 diabetes mellitus
Hyperlipidemia
Hypertension
Smoking (he had quit 8 years previously after a 12.5-pack-year history)
A remote history of alcoholism.
He also had end-stage kidney disease. He had received a kidney transplant 10 years before the current presentation but was back on dialysis because of transplant failure. He was still taking prednisone and tacrolimus.
He was also taking warfarin 2.5 mg, aspirin 81 mg, atorvastatin 80 mg, and insulin injections. He was not on any oral antidiabetic medications.
INITIAL EVALUATION
On initial physical examination, his right foot was edematous with extensive dry-appearing gangrene of the big toe, while the forefoot was relatively spared (Figure 1). We could feel no pedal pulses, the ankle-brachial and toe-brachial indices were low (see below), and pulse-volume waveform recordings demonstrated moderate dampening at the ankle and severe dampening at the level of the metatarsals and digits.
Notable laboratory and noninvasive vascular results at presentation
Resting right ankle-brachial index (ie, the systolic blood pressure in the ankle divided by the higher of the systolic pressures in the 2 arms) 0.51, compared with 0.64 1 month before (reference range 1.0–1.4)
Resting right toe-brachial index 0 (> 0.65)
Right wound, ischemia, and foot infection (WIfI) stage 4 (W-2, I-3, fI-0; more about this below)1
Hemoglobin concentration 11.0 g/dL (13–17 g/dL)
Mean corpuscular volume 84.2 fL (80–100 fL)
Mean corpuscular hemoglobin 25.3 pg (26–34 pg)
Mean corpuscular hemoglobin concentration 30.1 g/dL (30.5–36.0 g/dL)
Red blood cell distribution width-coefficient of variation 18.7% (11.5%–15.0%)
Serum creatinine 3.25 mg/dL (0.73–1.22 mg/dL)
Blood urea nitrogen 25 mg/dL (9–24 mg/dL)
Hemoglobin A1c 6.1% (4.3%–5.6%)
Low-density lipoprotein cholesterol (LDL-C) 80 mg/dL (< 100 mg/dL)
Computed tomography angiography was performed and later supplemented with catheter-based angiography to evaluate the arteries in his leg. The right superficial femoral artery had moderate focal stenosis, and there was severe infrapopliteal disease, with multilevel stenosis of the tibioperoneal trunk and total occlusion of the anterior tibial, posterior tibial, and peroneal arteries, all relatively close to their respective origins (Figure 2). Importantly, there was a short segment of the posterior tibial artery with a relatively normal vessel caliber that was reconstituted by collaterals at the supramalleolar level of the calf. No named vessels were identifiable distal to the malleolus.
Preoperative angiogram showing the patient’s (A) patent popliteal artery and (B) occluded posterior tibial artery (PTA).
Over the next month, the pain worsened, and the gangrenous toe became infected (fI-1) and needed to be amputated. A multidisciplinary team was convened to discuss the surgical options, consisting of specialists in internal medicine, cardiology, vascular surgery, podiatry, interventional cardiology, interventional and diagnostic radiology, and vascular medicine.
PERIPHERAL ARTERY DISEASE IS LINKED TO CARDIOVASCULAR DISEASE
1. For the moment, let’s put aside what needs to be done for the patient’s leg and think about his cardiovascular risk. Which of the following steps would be appropriate to improve it?
Perform echocardiography
Perform coronary angiography
Intensify his lipid-lowering therapy
Intensify his glycemic control
Patients with peripheral artery disease are at risk of concomitant atherosclerotic disease in other vascular beds, including the heart and brain. In a 2008 report of the Reduction of Atherothrombosis for Continued Health (REACH) registry,2 for example, about half of patients with peripheral artery disease also had coronary artery disease. This percentage is even higher in patients with chronic limb-threatening ischemia.
Further, the risk of major adverse cardiovascular events is significantly higher in patients with polyvascular disease. In the REACH registry, patients with symptomatic peripheral artery disease with polyvascular disease taking standard medications had rates of cardiovascular death, myocardial infarction, or stroke of 4.7% at 1 year and 9.1% at 2 years, and the rate of limb events was 5.7% at 2 years.3 The 3-year incidence rates of cardiovascular death, myocardial infarction, stroke, and repeat hospitalization were all significantly higher in those with polyvascular disease compared with those with involvement of a single vascular bed.4
This increased risk persists in more recent trials. In the placebo group of the 2017 FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) trial,5 the 3-year risk of major adverse cardiac events was about 17.4% in patients with peripheral artery disease with polyvascular bed involvement compared with 10% in those with peripheral artery disease alone.
Echocardiography and coronary angiography would not be indicated at this time, however. Despite the elevated risks, screening for coronary disease is not currently recommended in patients who have no coronary symptoms.6 This is because all patients with peripheral artery disease should receive intensive medical management. Further, we have no data to suggest that performing coronary revascularization before noncardiac arterial revascularization improves the cardiovascular outcomes of patients who have no coronary symptoms.
Intensive glycemic control can improve outcomes in patients with chronic limb-threatening ischemia. However, this patient’s hemoglobin A1c is already well controlled at 6.1%.7
More-intense lipid-lowering therapy should be considered for this patient. He has polyvascular atherosclerotic disease, prior cardiovascular events, and chronic limb-threatening ischemia. His LDL-C level of 80 mg/dL at presentation is within the reference range for the general population, but for someone with his history it should be lower—he is still at “very high risk” for recurrent events and therefore would benefit from adding an adjunctive agent such as ezetimibe, a proprotein convertase subtilisin/kexin type 9 inhibitor, or both if needed, with a target LDL-C level lower than 55 mg/dL.8,9 Just before his intervention, our patient’s LDL-C was 34 mg/dL, with no adjunctive agents.
OTHER RISK FACTORS FOR PERIPHERAL ARTERY DISEASE
Many other factors pertinent to our patient affect the risk and outcomes of peripheral artery disease, including social and economic determinants of health and modifiable risk factors. The most significant risk factors involved in this patient’s presentation, management, and recovery were diabetes mellitus and chronic kidney disease. Furthermore, Black people, as evidenced in our patient, have been shown to be at higher risk for chronic limb-threatening ischemia and undergoing amputations.10 This is due to unequal access to care and socioeconomic inequalities that contribute to inadequate management of the aforementioned risk factors.
Diabetes mellitus is an independent risk factor for amputation due to infection and peripheral neuropathy, the latter of which results in diabetic ulcers and foot deformities.11 Concomitant peripheral artery disease amplifies such risk by impairing arterial inflow and wound healing. Patients with peripheral artery disease with diabetes mellitus are more likely to develop chronic limb-threatening ischemia and undergo amputation compared with their counterparts without diabetes.12 Those patients are further burdened with higher mortality rates at a significantly younger age compared with patients with peripheral artery disease who do not have diabetes.12
Chronic kidney disease. The prevalence of peripheral artery disease is higher in patients with chronic kidney disease than in the general population, and its prevalence increases with increasing severity of the kidney disease.13 Furthermore, the severity of peripheral artery disease correlates with the severity of chronic kidney disease.14 Chronic kidney disease is also a factor in the outcomes of peripheral artery disease and revascularization procedures; it independently increases the risk of death and limb loss after revascularization, particularly in patients with end-stage kidney disease.15–17
HOW SHOULD WE MANAGE HIS LIMB ISCHEMIA?
2. Which of the following is the best option for managing our patient’s peripheral vascular disease at this point?
Amputation of his foot
Open arterial bypass surgery
Endovascular arterial revascularization
Deep venous arterialization
Global guidelines on management of chronic limb-threatening ischemia call for assessing 3 factors when considering revascularization procedures: the patient’s cardiovascular risk (to determine whether they can undergo surgery without suffering a major adverse cardiovascular event), the stage of the peripheral vascular disease (limb staging, to determine whether they need to undergo surgery), and the anatomic pattern of disease (to determine whether and how surgery can be done).18
Preoperative cardiovascular risk stratification
Perioperative cardiac risks with peripheral vascular disease surgery are determined by patient-related factors and the type of surgery.
Patients undergoing peripheral artery revascularization are at moderate to high risk of perioperative adverse cardiac events such as nonfatal myocardial infarction or cardiac death.19 In the National Surgical Quality Improvement Program study, major adverse cardiac events occurred in 2.0% of 2,155 patients undergoing lower-extremity bypasses to treat claudication symptoms only, and in 1.0% of 1,770 patients undergoing infrainguinal endovascular interventions.20 In another study, the rate of cardiac complications was higher in 580 patients with chronic limb-threatening ischemia, ranging from 1.3% to 2.1% for acute myocardial infarction and 3.0% to 3.8% for perioperative mortality.21 Therefore, it is imperative to address any potential reversible risk factors.
Perioperative cardiac risk evaluation begins with a focused cardiovascular history and physical examination. It is also reasonable to obtain an electrocardiogram for most patients. Any unexplained cardiovascular symptoms (eg, dyspnea, chest pain, or syncope), abnormal examination findings (eg, new murmur, jugular venous distension, or pedal edema), or worrisome electrocardiographic abnormalities (eg, advanced conduction disease, newly diagnosed pathologic Q waves) may warrant additional investigations that may include chest radiographs, echocardiography, or ischemia testing.
If nothing worrisome is noted, several risk assessment tools can be used to estimate the patient’s perioperative risk of major adverse cardiac events, such as the revised cardiac risk index, the National Surgical Quality Improvement Program risk calculator, and the Vascular Study Group cardiac risk index.22 However, most patients will be at intermediate to high risk. Routinely measuring cardiac biomarkers (high-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide) can also provide additional prognostic information in patients without symptoms undergoing intermediate- or high-risk surgery, and is recommended by the European guidelines,23 but not by the American guidelines.24
For patients who cannot exercise at more than 4 metabolic equivalents—and most patients with chronic limb-threatening ischemia cannot—it is reasonable to consider a pharmacologic stress test (nuclear vs echocardiogram) before any intermediate- or high-risk procedures. Evidence of moderate- to large-territory ischemia or severely depressed left ventricular ejection fraction may warrant coronary angiography before the procedure.
Of note, while routine coronary revascularization has never been shown to improve perioperative cardiovascular outcomes, decisions about revascularization are made on a case-by-case basis based on standard revascularization guidelines.25 Higher-risk lesions such as multivessel coronary disease or left main disease will need additional considerations based on the risks of delaying coronary vs peripheral artery intervention. A team-based multidisciplinary approach is critical to achieving good patient outcomes.26
Despite our patient’s significant history of cardiovascular disease, electrocardiography indicated left axis deviation but no pathologic Q waves. Echocardiography revealed normal left ventricular systolic function with an ejection fraction of 65% ± 5% (2-dimensional biplane) and no valvular dysfunction. A cardiac stress test was unremarkable with normal ST-segment response, and angina was not provoked. A cardiac nuclear stress test demonstrated normal perfusion with a reduced ejection fraction of 45%. Thus, we decided he could proceed with his surgery.10
Limb staging
Limb staging uses the “WIfI” classification system,1 which assigns up to 3 points each for the wound (W), ischemia (I), and foot infection (fI). The patient’s right limb had a gangrenous digit (W-2), severe ischemia (I-3), and mild infection (fI-1), consistent with WIfI stage 4, the highest. This means he was at high risk of amputation unless we attempted to revascularize his foot.
The anatomic pattern
Thus, our patient needed surgery to save his foot, and he was able to undergo surgery from a cardiac standpoint. But could we actually do anything for him?
Our patient had multilevel occlusive disease. He had only moderate stenosis of the superficial femoral artery stenosis that was less than 10 cm and no significant disease in the popliteal artery. However, the tibioperoneal trunk was severely narrowed, all 3 infrapopliteal vessels were chronically occluded and severely calcified, and there was no inframalleolar target artery crossing the ankle into the foot.
Given the advanced limb stage and lack of a pedal or plantar target artery, our patient had no options for distal arterial bypass. Criteria of no-option anatomy are “desert” foot, defined as no patent pedal arteries, or inadequate venous conduit for bypass due to severe calcification or long-segment occlusion.27 This challenging situation occurs in up to 20% of patients with chronic limb-threatening ischemia.
THE PATIENT WANTS TO KEEP HIS FOOT
Our patient was at extremely high risk of losing his foot if we did nothing, and with no arteries available for revascularization, amputation might have been a reasonable option at this point. However, after a comprehensive discussion with the patient and his wife, he adamantly declined this option. Therefore, we decided to explore other revascularization options.
Currently, there are no guidelines or adequate data comparing the relative efficacy of alternative treatments for patients with no-option anatomy, but one of them is deep venous arterialization.
DEEP VENOUS ARTERIALIZATION: AN OPTION WHEN THERE IS NO OPTION
Deep venous arterialization is an option in cases in which no inframalleolar target artery path is available for conventional revascularization, as in our patient. It involves directing arterial blood flow to a deep vein via a conduit such as an autogenous vein graft (Figure 3).
(A) Angiogram of popliteal-to-posterior tibial artery bypass using a reversed greater saphenous vein (rGSV) graft. (B) Venogram of rGSV-to-posterior tibial vein bypass. (C) A drawing shows deep venous arterialization of the posterior tibial vein.
This procedure can be performed using an open approach, a percutaneous approach, or a novel hybrid approach, but we expect that newer specialized endovascular devices will lead to wider use of less-invasive approaches. In our patient, open bypass was selected as the planned first stage in view of his anatomic occlusive pattern. Open tibial artery bypass with an autogenous conduit has demonstrated superior patency compared with endovascular tibial intervention.
Acceptable outcomes have been described for both the open and percutaneous approach; however, no direct randomized comparisons have been performed for these techniques. A literature review from 2020 showed that the open approach had better patency rates; however, few studies directly compared the open and percutaneous procedures, making it hard to make evidence-based clinical decisions.28 Possible reasons for better patency rates with open bypass surgery are the ability to directly ligate perforating veins and reverse the vein to eliminate significant flow disruption from the residual obliterated venous valve, which can cause early graft failure.29,30
Outcomes of deep venous arterialization
Published patency rates of deep venous arterialization for chronic limb-threatening ischemia are 44% to 88% at 1 year with the open approach, 29% to 40% at 6 months with the endovascular approach, and 6.9% at 1 year with the hybrid approach.31–33 Major amputation rates range from 0% to 70% with the open approach, 0% to 28.5% with the endovascular approach, and 23% to 31% with the hybrid approach.28
These comparisons are limited by the paucity of studies, their retrospective nature, and their substantially heterogeneous populations. Nevertheless, given the current evidence, open deep vein arterialization is an option with acceptable efficacy for patients in whom major amputation would be the only other option.
Techniques of deep venous arterialization
LimFlow is a novel endovascular system that uses an arterial and a venous catheter, which are placed under ultrasonography guidance to obtain better alignment, crossing, and retrieval of the wire, after which stent grafts are deployed.33 A multicenter trial (Percutaneous Deep Vein Arterialization for the Treatment of Late-Stage Chronic Limb-Threatening Ischemia [PROMISE II]) of this system is underway in 20 sites across the United States with a goal of enrolling 100 participants. Preliminary 6-month results in 105 patients were promising, with an amputation-free survival rate of 66%, significantly exceeding the target endpoint of 54%. Furthermore, the limb salvage rate was 76%, the survival rate was 87%, and the wound healing rate was 76%.34
In centers where the commercially manufactured device is not available or reimbursed, off-the-shelf items can be used as an alternative approach. Several techniques have been described in performing off-the-shelf techniques—the arteriovenous spear technique, the venous arterialization simplified technique, and use of a penetration wire or reentry device.
The arteriovenous spear technique is performed by simultaneously puncturing the tibial artery and vein under duplex ultrasonography visualization.35 This technique does not require a snare or balloon for vessel wall penetration. The limitation of this technique is it relies heavily on the technical skill in the puncturing process.
The venous arterialization simplified technique uses an overlapping inflated balloon and snare catheter to insert a needle under a fluoroscopic view.36 However, small, tortuous, and calcified vessels, particularly in below-the-ankle arteries, make it more challenging to pass the snare catheter. A study in 18 patients in Japan assessed 12-month outcomes using the combination of arteriovenous spear technique and the venous arterialization simplified technique.37 The technical success rate was 88.9%, the limb salvage rate was 72.2%, and the amputation-free survival rate at 12 months was 49.4%.
The use of a reentry device or penetration wire with a heavy tip is limited by the difficulty of penetrating the vessel wall if it is heavily calcified. The alternative step is to use a posterior tibial artery balloon to expand the target punctured vessel. In a case series of 14 patients who underwent the procedure with intravascular ultrasonography guidance, the technical success rate was 100%, the median time of primary patency was 8 months, and the limb salvage rate was 78% within 2 years of follow-up.38
CASE CONTINUED: SURGERY AND POSTOPERATIVE COURSE
We performed open deep venous arterialization, using the greater saphenous vein as a graft to link the popliteal artery, the posterior tibial artery, and the posterior vein by end-to-side anastomosis and ligating all the side branches to the posterior tibial vein (Figure 3). In a subsequent procedure, we performed endovascular vein valve lysis of the tibial and plantar venous arch to complete the pedal revascularization.
A pulse was palpable in the bypass graft at the end of the procedure. Postoperative imaging showed the bypasses were patent and outflow to the foot via the arterialized deep venous and plantar arch system was significantly improved. The patient tolerated the procedure well and recovered appropriately.
ANTITHROMBOTIC REGIMENS
3. What is the recommended postoperative antithrombotic regimen for this patient?
Rivaroxaban 2.5 mg twice a day with aspirin
Full-dose anticoagulation therapy alone
Warfarin (target international normalized ratio 2.5) with an antiplatelet agent
Aspirin or clopidogrel alone
After arterial bypass, we need to consider the risk of thrombosis in both the bypass target vessel (taking into account its caliber and quality) and the conduit used (autogenous vs prosthetic). In this patient, the runoff was deemed “disadvantaged” as a result of both size and caliber. For this reason, long-term anticoagulation (warfarin or an oral antithrombotic) is indicated.39 Our patient was discharged home taking warfarin (with a target international normalized ratio of 2.5), and continued to take aspirin 81 mg once daily.
There are data to support the use of rivaroxaban 2.5 mg twice a day along with aspirin 81 mg for patients with peripheral artery disease after lower limb revascularization, but rivaroxaban is contraindicated in patients with advanced renal disease.40–42 The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial43 compared the postoperative use of rivaroxaban (with or without aspirin) vs aspirin alone in patients with stable atherosclerotic disease. Those who were on rivaroxaban had fewer cerebrovascular and cardiovascular events with comparable major bleeding complications.
Similar findings were reported in the subsequent VOYAGER PAD (Vascular Outcomes Study of ASA Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization for Peripheral Artery Disease) trial,44 which compared rivaroxaban with aspirin and aspirin alone following lower-limb revascularization. Compared with those on aspirin alone, patients taking rivaroxaban 2.5 mg twice daily along with aspirin had significantly lower rates of major adverse cardiovascular events (myocardial infarction, ischemic stroke, death) and lower-limb events (acute limb ischemia, major amputation) (15.5% vs 17.8%; P = .009). The risk of major bleeding was similar between the 2 groups as assessed by the Thrombolysis in Myocardial Infarction grading system (P = .07); however, it was higher with rivaroxaban and aspirin than with aspirin alone according to the International Society on Thrombosis and Haemostasis grading system (P = .007).
CASE CONCLUDED
At the patient’s first follow-up visit, his right ankle-brachial index had improved from 0.51 previously to 0.73, with normal pulse-volume waveforms at the ankle. Chronic Pseudomonas osteomyelitis, diagnosed by microbiological testing of tissue and bone, hindered wound healing, necessitating a transmetatarsal amputation. Six weeks after surgery, the patient underwent catheter-based intervention of the venous system to obliterate valve structures and augment outflow. His ischemic pain had resolved, and the amputation site had healed.
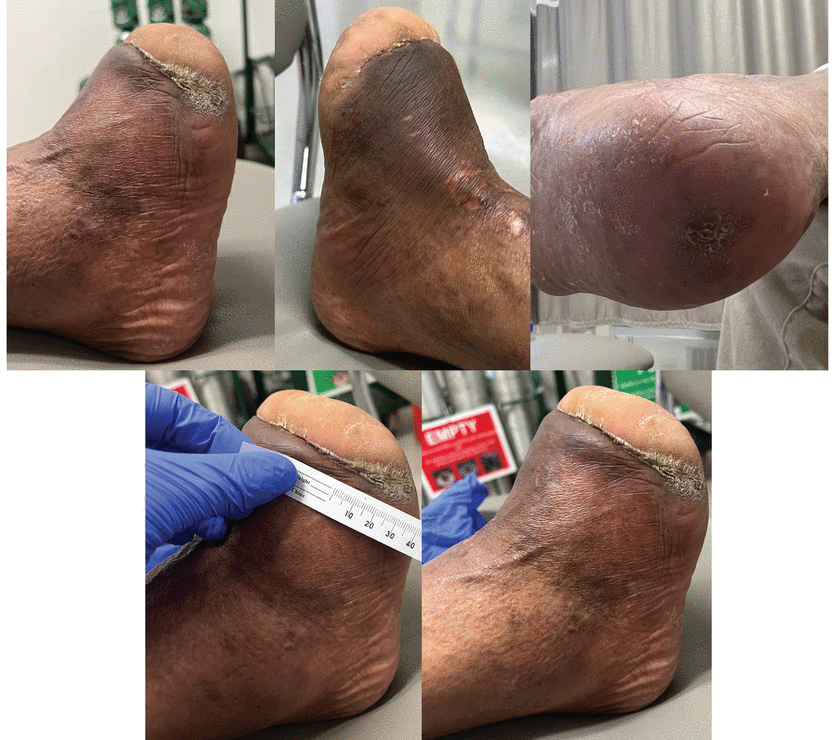
Two years after surgery, the patient was doing well, his pulse-volume recordings were unchanged, and the arterial bypass and deep venous system were still patent (Figure 4). He is ambulatory in diabetic shoe wear. He is currently off antibiotics and is maintained on appropriate blood-thinning medications.
Healed right foot 2.5 years after surgery.
This case shows that deep venous arterialization can be a viable revascularization option for high-risk patients with advanced chronic limb-threatening ischemia and a “no-option” anatomic arterial occlusive pattern. As with all patients who have undergone revascularization for chronic limb-threatening ischemia, close surveillance with primary-assisted procedures can play a role in prolonging patency. Additionally, a multidisciplinary approach and patient-centered care are crucial to achieving favorable outcomes in limb-threatened patients with advanced disease. This includes thorough preoperative preparation, selecting the appropriate surgical intervention, and optimal postoperative medical therapy.
TAKE-HOME POINTS
The aim of treating chronic limb-threatening ischemia is to restore blood flow to the region of tissue loss to permit complete wound healing and to return the patient to ambulatory status. In this patient population, the WIfI classification stratifies the risk of amputation and the potential benefit of revascularization.
The finding of peripheral artery disease represents an opportunity to initiate and optimize guideline-directed medical therapy and reduce the patient’s risk of major cardiovascular and cerebrovascular events.
Revascularization may be accomplished by open bypass surgery or catheter-based intervention depending on multiple factors, such as the presence of rest pain or tissue loss, medical comorbidity profile, the presence of saphenous vein conduit, and the anatomic distribution of the arterial occlusive process.
Patients in whom conventional arterial bypass or endovascular revascularization is not technically feasible have what is referred to as a “no-option” arterial occlusive anatomic pattern. For those patients, major limb amputation at a below-the-knee level is the only plausible option by conventional management strategies.
In selected cases, deep venous arterialization can be a viable last-resort option for revascularization for those with advanced chronic limb-threatening ischemia and a “no-option” anatomic pattern before considering major amputation. Clinical research is ongoing to help define the patient profile with the greatest benefit relative to risk.
Primary patency and amputation rates vary following open, endovascular, and hybrid deep venous arterialization.
A multidisciplinary approach and patient-centered care are crucial to achieving favorable outcomes in limb-threatened patients with advanced disease. The interdisciplinary approach is necessary in preoperative preparation, selection of the appropriate revascularization strategy, and optimal post-operative medical therapy.
DISCLOSURES
The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.