In 2016, laboratory tests to detect vitamin D deficiency were ordered more than 10 million times for Medicare patients, up 547% since 2007, at a cost of $365 million.1 In 2017, sales of vitamin D supplements totaled $936 million, a 9-fold increase over the previous decade,1 and expected to rise to $1.3 billion by 2025, for an annual growth rate of 5.8% from 2020 to 2025.2 This astronomic increase in vitamin D testing and supplementation is happening in the absence of any real evidence-based rationale.
See related editorial, page 91
UNCERTAINTY OF EVALUATING VITAMIN D STATUS
Evaluation of vitamin D status has long been problematic and plagued with confusion. At first, it was somewhat unclear as to which blood test—1,25-dihydroxyvitamin D or 25-hydroxyvitamin D (25[OH]D)—is the most informative. After 25(OH) D was settled on,3 debate ensued over which blood levels are the most informative in assessing vitamin D status and what the cutoff points should be. It was concluded that the terms insufficiency and deficiency could be distinguished from one another, although they seem much the same to many clinicians. Even experts in the field of bone health cannot agree on which levels are acceptable (Table 1).3,4
Recommendations for deficiency and inadequacy of 25-hydroxyvitamin D
IS VITAMIN D DEFICIENCY OR INSUFFICIENCY TRULY A PANDEMIC?
The prevalence of vitamin D deficiency is considered to be remarkably high: 41.6% of American adults had serum 25(OH)D levels below 20 ng/mL (50 nmol/L) in 2011,5 levels considered to be consistent with vitamin D deficiency. The prevalence is high enough to be dubbed pandemic by some authors.6 Worldwide, it has been estimated that 1 billion people have vitamin D deficiency or insufficiency,7 which many find difficult to believe.7 Much of this confusion is caused by the presumption that serum levels of 25(OH)D reflect nothing but vitamin D status. But is it possible that the levels are influenced by something else?
THE CASE FOR VITAMIN D AS A NEGATIVE ACUTE-PHASE REACTANT
The short answer is yes, there is compelling evidence that 25(OH)D is a negative acute-phase reactant—its serum levels decrease in the presence of inflammatory states.8–11 Several lines of evidence support this conclusion:
Serum C-reactive protein and 25(OH)D levels are inversely associated, as would be expected if 25(OH)D were a negative acute-phase reactant.12–16 In quantitative terms, the inverse relation between 25(OH)D below its median and C-reactive protein levels was found to be significant: a geometric mean change in C-reactive protein of 0.11 mg/dL for each 10-ng/mL change in 25(OH)D (95% confidence interval 0.16 to −0.04) on multivariate linear regression analysis.12
Low blood levels of 25(OH)D have repeatedly been found to be associated with a variety of inflammatory states.17–26
Most tellingly, 25(OH)D levels fall after a variety of inflammatory insults, a classic test for acute-phase reactant behavior.9,10,27 A surgical procedure, an induced inflammatory insult, may be associated with a 40% reduction in circulating 25(OH)D levels when compared with preoperative values.28
Low levels of 25(OH)D found in patients with obesity persist despite various aggressive vitamin D supplementation regimens, as would be expected of a negative acute-phase reactant.29
THE ACUTE-PHASE RESPONSE
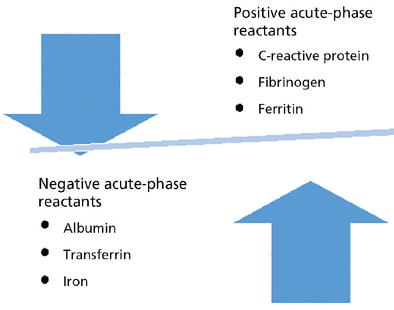
The acute-phase response refers to a large number of behavioral, physiologic, biochemical, and nutritional changes that occur during inflammatory states. Figure 1 shows examples of positive and negative acute-phase reactants.30 A 1999 review reported that C-reactive protein and fibrinogen are prototypical positive acute-phase proteins whose plasma concentrations increase during inflammatory states, whereas albumin and transferrin are negative acute-phase proteins whose concentrations decrease.30 Although the review largely focused on acute-phase proteins, the other components of the systemic response to inflammation should not be forgotten. Cations may also display acute-phase behavior. Examples include a decrease in concentrations of zinc and iron and an increase in copper concentration. Most significant for our purposes is research documenting the negative acute-phase behavior of a variety of vitamins.31 This has been problematic for investigators and clinicians because the acute-phase behavior of these molecules tends to be overlooked. It has been noted that misclassification of vitamin A status can occur because serum retinol levels decrease during the acute-phase response.32
Examples of positive and negative acute-phase reactants.
Based on information in reference 30.
Similar problems are raised by other acute-phase reactants. Low serum albumin levels are often taken as evidence of malnutrition, although the low levels frequently reflect albumin’s behavior as a negative acute-phase reactant. A similar tale can be told about iron. While low serum iron levels may indicate iron deficiency, they may instead reflect an underlying inflammatory process. Clinicians are aided by the fact that transferrin, usually estimated by total iron binding capacity, is a negative acute-phase reactant. When low transferrin levels are found, it suggests the presence of an inflammatory process, whereas elevated transferrin values are usually seen in iron deficiency.
WHAT IS MEANT BY INFLAMMATION?
It is common for patients to ask us, “What can I do to lower my inflammation?” We should not be surprised by this. Patients are inundated with media reports that inform them that they can “fight inflammation” based on the premise that inflammation constitutes a single malicious process in the body. In fact, inflammation, a widely abused term, is not at all a simple process. It is a complex biological cascade that may involve, to varying degrees, a number of different cell types as well as multiple cytokines, histamines, bradykinin, prostaglandins, leukotrienes, platelet-activating factor, complement components, inflammasomes, and a family of molecules that promote cell adhesion. It is important that clinicians be aware of the complexity of these processes and impart that information to their patients.
Inflammation has classically been defined as a defense mechanism against infection and tissue injury, employing the innate immune response to localize and eliminate injurious factors and remove damaged tissue components. Its ultimate purpose is to return tissues to their normal state. A large number of medical conditions (eg, cardiovascular disease, obesity, type 2 diabetes), however, have been found to be associated with components of the inflammatory response, in the absence of infection or tissue injury. While it is generally presumed that inflammation participates in the pathogenesis of these conditions, it is equally likely that metabolic perturbations induce inflammation. Indeed, it has become apparent in the last decades that low-grade inflammation can be induced by tissue stress and malfunction (“metaflammation”), by changes from the optimal internal environment and the absence of infection or overt tissue injury.33,34
LOW-GRADE INFLAMMATION
Low-grade inflammation (metaflammation) differs from the inflammation resulting from infection or tissue injury. It is not accompanied by the 4 classic signs of inflammation—rubor (redness), tumor (swelling), calor (warmth), and dolor (pain)—and manifests only minor degrees of C-reactive protein elevation, commonly regarded as an indicator of the presence of inflammation. While the purposes of classic inflammation are defense, healing, and tissue repair, the purpose of low-grade inflammation is the restoration of normal homeostasis. Acute inflammation is largely triggered by the pattern-recognition molecules PAMPs (pathogen-associated molecular patterns) and DAMPs (damage-associated molecular patterns), while low-grade inflammation is triggered by sentinel cells that monitor for deviations from the optimal homeostatic state.35,36
Low-grade inflammation is not rare. Modest C-reactive protein elevation, defined as concentrations between 3 and 10 mg/L, has been documented in approximately 30% of the US population.37 Low-grade inflammation, manifested by modest C-reactive protein elevation, is associated with an astounding number of conditions and lifestyles, most of which are associated with poor health. These conditions represent or reflect minor metabolic perturbations, capable of inducing metaflammation. A partial list includes obesity, diabetes, atrial fibrillation, obstructive sleep apnea, hypertension, prehypertension, sleep deprivation, low levels of physical activity, lumbar disc herniation, polycystic ovary syndrome, various unhealthy diets, hypoxia, social isolation, and aging, as well as smoking and exposure to environmental irritants such as second-hand smoke.38
WHY SO MUCH INTEREST IN VITAMIN D?
It is well established that vitamin D is essential for skeletal health, but in recent years, evidence has been presented purporting to show that it plays a critical role in host defense39 and in modulating both innate and adaptive immune responses.40 It has been proposed that vitamin D administration inhibits inflammation and lowers the incidence of cancer and cardiovascular events.41 Attempts have been made to link inadequate vitamin D levels to high susceptibility to chronic infections and to autoimmune diseases. Observational studies have found associations between low vitamin D levels and the risk of fractures, falls, mortality, diabetes, hypertension, and a variety of other disorders,42,43 and a 2022 systematic review that found that patients with severe COVID-19 infection had lower levels of 25(OH)D than patients with milder infection.44
Based on the assumption that low 25(OH)D levels reflect nothing but less-than-optimal vitamin D status, clinical trials have been conducted, and more are in progress, to determine whether vitamin D supplementation can reduce the likelihood that these conditions will occur or can avert severe disease associated with COVID-19. However, a nationwide, randomized, placebo-controlled trial found that supplementation with vitamin D did not result in a lower incidence of invasive cancer or cardiovascular events than placebo,45 a conclusion supported by other investigators who have similarly reported that vitamin D supplementation did not lead to significant reduction in all-cause mortality or mortality from cancer and cardiovascular disease.46,47 And no significant difference has been found in major health-related outcomes in COVID-19 with vitamin-D supplementation.48,49
Are such clinical trials justified? One might argue that it is appropriate research, as there is much interest in the topic, and we do not have definitive answers. True. But the scientific rationale for carrying out such studies is undermined somewhat by the fact that vitamin D is a negative acute-phase reactant and that low levels of 25(OH)D may merely reflect metabolic perturbations.
THE SOCIETAL COST OF TOO MUCH CURIOSITY ABOUT VITAMIN D
As noted earlier, the increase in vitamin D testing and supplementation in the absence of a strong evidence base leads to an accelerating rise in economic costs. The Choosing Wisely Canada program50 recommends checking serum 25(OH)D levels in patients with only a few select medical conditions (osteoporosis, inflammatory bowel disease, celiac disease, kidney and liver disease, and pancreatitis) and recommends against testing in the general population. The Choosing Wisely campaign of the American Society for Clinical Pathology51 also recommends against population-based vitamin D testing and recommends testing only in similar select populations. However, it states that laboratory testing is appropriate in higher-risk patients when results will be used to institute more aggressive therapy (eg, osteoporosis, chronic kidney disease, malabsorption, some infections, obesity).51
WHEN SHOULD WE RECOMMEND VITAMIN D SUPPLEMENTATION?
The high prevalence of low-grade inflammation in the general population argues against reflexively concluding that some degree of insufficiency or deficiency of vitamin D is present when a decreased concentration of serum 25(OH)D is found. Thus, finding a low vitamin D level in a patient whose C-reactive protein level is not elevated supports the possibility of vitamin D deficiency. However, finding an elevated C-reactive protein concentration or low albumin level is consistent with the possibility that systemic inflammation underlies the depressed 25(OH)D level, as well as the possibility that both vitamin D deficiency and systemic inflammation are present. In addition, the recent finding that the analytical performance of immunoassays for 25(OH)D is highly variable further complicates the interpretation of laboratory test results.52 All of this argues, of course, against routinely prescribing vitamin D supplements, even when low 25(OH)D levels are found.
THE NEXUS OF INFLAMMATION AND VITAMIN D: WHAT A MESS!
Much uncertainly lies in when to evaluate vitamin D, in the reliability of assays, in the significance of various 25(OH)D levels, and in the level of true deficiency. Often overlooked is the recognition that 25(OH)D levels may be low in the presence of both acute and low-grade inflammation and may represent a true nutritional deficiency. Despite expert guidance on when to determine vitamin D levels, many practicing clinicians are pressured into inappropriate ordering of this test and repleting “low” levels. We encourage conversations between clinicians and their patients regarding vitamin D testing and supplementation.
DISCLOSURES
Dr. Epstein has reported consulting for Bayer Healthcare. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Acknowledgment
We are grateful to Nathan Berger, MD, for his helpful comments.
- Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.