ABSTRACT
Increasing numbers of patients with cardiac disease have improved quality of life and longevity as a result of cardiac implantable electronic devices (CIEDs). CIED infections can involve the generator pocket, bloodstream, or cardiac structures and occur in about 0.5% of de novo CIED implants and approximately 2% of CIED replacements. Prompt diagnosis of CIED infection is beneficial to the success of antibiotic therapy and subsequent device removal to resolve the infection. Measures to prevent CIED infections include assessment of the indication and patient status, strict sterile surgical techniques, preoperative antibiotics, and adequate homeostasis. New surgical methods and CIED devices may also lead to reduction in CIED infections. Further research is needed to better quantify the incidence of CIED, risk factors, and efficacy of surgical techniques to prevent infections.
CIED use is increasing, as are the number of CIED infections, which are associated with significant morbidity and mortality.
Prompt diagnosis of CIED infection allows for early management with antibiotics and device removal, which is typically needed for resolution of the infection.
Prevention of CIED infection is an important strategy, and more research is needed to inform the incidence of CIED infection, risk factors, and devices and techniques to minimize the risk of infection.
Cardiac implantable electronic devices (CIEDs) have become common tools to improve the quality of life and longevity of patients with cardiac disease over the last few decades.1–4 CIEDs include implantable cardioverter defibrillators (ICDs), permanent pacemakers, biventricular pacemakers providing cardiac resynchronization therapy with or without a defibrillator, subcutaneous ICDs, and implantable loop recorders. With increasing approved indications, the number of CIEDs implanted each year continues to grow. This, paired with the aging population of patients receiving devices and their medical complexity, has led to a corresponding increase in device-related complications.2,3 One of the most serious complications is CIED infection, which leads to significant morbidity and death. These infections also represent a significant cost burden to the healthcare system, with treatment costs for a CIED infection estimated at over $146,000 in 2008.5
SCOPE OF THE PROBLEM
More than half a million permanent pacemakers and ICDs are implanted each year in the United States, with more than 4 million implanted between 1993 and 2008.5 The risk of infection is 0.5% to 1%, for a first-time implantation and 1% to 5% for a device replacement or upgrade.1,2,5–9 These infections can involve the generator pocket, bloodstream, or cardiac structures, leading to infective endocarditis.10 The timing of CIED infection appears to be bimodal in distribution: early infections usually occur as a result of the implantation procedure itself, whereas late infections occur in patients who are generally unwell or because of an insidious process that eventually crosses a threshold of clinical significance.3,11,12
Incidence and risk factors
Klug et al13 investigated the incidence rate and risk factors of CIED infection prospectively in a large cohort of patients from 44 centers who underwent CIED implantation. Of 6,319 procedures, 4,465 were first implants and the other 1,854 were a replacement or revision; 42 patients (0.68%) developed CIED infection by 12 months after the procedure, and the incidence of infection in replacement or revision cases was nearly twice the rate found in first implants.13 Risk factors for CIED infection included renal failure, heart failure, diabetes, and fever within last 24 hours before CIED implantation.14 The Implantable Cardiac Pulse Generator Replacement (REPLACE) registry found the 6-month incidence rate of CIED infection to be 1.4% after CIED replacement.6
Recently, there has been concern that the rate of newly infected CIEDs has outpaced the rate of newly implanted ones.5,15 Voigt et al15 reported a 12% increase in the rate of CIED implantation from 2004 to 2006 and an out-of-proportion 57% increase in the rate of CIED infection. A review from 2011 confirmed these findings, showing the annual CIED implantation incidence increased an average of 4.7% per year between 1993 and 2008.5 This was probably driven by clinical trials that broadened the indications for ICD implantation for primary prevention.16–19 Between 1993 and 2008, the rate of newly implanted devices increased by 96%, while the rate for newly infected CIEDs increased by 210%; the majority of this increase occurred after 2004.5 The study showed that comorbidities in patients receiving CIEDs increased sharply starting in 2004—alluding to the contribution of comorbid medical conditions such as renal failure, respiratory failure, heart failure, and diabetes to infection risk.5
However, a major obstacle to defining the true incidence rate of CIED infection is the lack of a clear denominator. CIED infection is not limited to the first few months after implantation. In fact, over half of these patients present more than 1 year after the last CIED intervention.12 Therefore, the number of patients at risk continues to grow each year and includes patients who underwent implantation that year or before, making it very difficult to compare infection rates. Additionally, the lack of a clear definition of CIED infection and the variations in duration of follow-up in different studies make it difficult to accurately assess the incidence of CIED infection.
PATHOGENESIS
A CIED can become infected at the time of implantation or pocket revision. The infection can then track along the endovascular portion of the leads resulting in endovascular infection and possibly endocarditis. A CIED can also become infected as a result of the hematogenous seeding of the leads or pocket during an episode of bacteremia. Most of these infections (70%) are caused by staphylococcal species, and many are becoming resistant to methicillin.12 Other species include gram-negative organisms (9%), enterococci (4.2%), streptococci (2.5%), and fungi (1%) (Table 1). Despite clear evidence of clinical CIED infection, the cultures remain negative in about 13% of cases, perhaps because of the unfortunately common prac-tice of starting antibiotic therapy before obtaining cultures or because of the need to incubate culture samples for a longer duration.12 A longer incubation time is particularly important for infections involving Proprionibacterium acnes, an aerobic gram-positive rod commonly associated with acne vulgaris.20
Pathogens identified in 816 patients with lead extraction or device removal for CIED infection
DIAGNOSIS
Prompt and accurate diagnosis of CIED infection is critical as it allows for early management with antibiotic therapy and device removal. As the number of CIED implantations increases, providers on the front lines—emergency, family practice, and internal medicine physicians—will play an increasing role in recognizing and diagnosing CIED infection. Patients with CIED infection present with a range of signs and symptoms including fever, chills, erythema, swelling, drainage, tenderness, malaise, erosion, and warmth of the skin overlying the generator pocket.2 In 55% of cases, patients present with localized pocket infection, while the remaining patients have signs of an endovascular infection without obvious pocket involvement.12 Localized pocket infection is more common during the first year after device implantation. CIED-associated endovascular infections occur more commonly in patients with multiple comorbidities including diabetes, renal failure, prior heart valve operation, rheumatic heart disease, and prior bloodstream infection.2 Despite the theoretical divide in CIED infections (endovascular vs pocket), overlap is common: many patients with pocket infection show evidence of bacteremia and vegetations on the leads.
Physical examination of the pocket is critical as it may reveal visible signs of infection and support the diagnosis of localized pocket infection (Figure 1). Blood cultures are essential and should be collected before starting antibiotic therapy. Culture results assist in the diagnosis of CIED infection and also help identify the microorganism involved, and this information helps tailor the choice and duration of antibiotic therapy. Echocardiography (transthoracic and transesophageal) can assist the clinician in the diagnosis of CIED infection but requires careful interpretation because some patients with no signs or symptoms of infection can have small fibrinous strands or thrombi attached to the CIED leads.14 These findings should only be interpreted in correlation to the clinical presentation.
Pocket infection after placement of a cardiac implantable electronic device can present as erythema and drainage (A); swelling, skin necrosis, and eschar formation (B); and erythema, swelling, and bullae formation (C).
Diagnosing pocket infection from the physical examination can be difficult due to the often subtle manifestations of the underlying pathophysiology and because visible changes to the pocket can occur over weeks and months. Furthermore, differentiating superficial infection, hematoma, seroma, and allergic reactions from deep pocket infection can be challenging. In cases when the diagnosis is not clear and there are no systemic findings of infection, conservative management with close follow-up is reasonable. Similarly, the diagnosis of endovascular infection is sometimes delayed because the symptoms are not very specific or because of a lack of awareness of the presence of a CIED and its role in endovascular infection.
MANAGEMENT
A multidisciplinary approach involving cardiology, infectious disease, electrophysiology, and cardiothoracic surgery teams is required to optimize outcomes in patients with CIED infection. CIED infection is particularly difficult to treat with antibiotic therapy alone because it involves infection of an implanted device and an associated biofilm that is resistant to the effects of antibiotics. Once infection is confirmed, antibiotic therapy serves as an adjunct to the complete removal of the hardware. Most patients receive 2 weeks of intravenous antibiotics after removal of an infected CIED, with longer courses for patients with Staphylococcus aureus infection or documented endocarditis.21
Infectious disease consultation is paramount in order to choose the appropriate type and duration of antibiotic therapy. Conservative approaches that involve using antibiotics alone or incomplete system removal have high failure rates with high rates of morbidity and mortality.13,21–28 However, chronic antibiotic suppressive therapy may be considered as a palliative measure for patients who are not candidates for lead extraction.
DEVICE REMOVAL
Confirmation of CIED infection is a class I indication for device removal and the patient should be referred to an electrophysiologist. Transvenous lead extraction (TLE) is a percutaneous procedure performed by the electrophysiologist in the electrophysiology laboratory or hybrid operating room with cardiothoracic surgery support, and it is generally performed under general anesthesia with invasive hemodynamic monitoring. After opening and debriding the infected pocket, the generator is disconnected from the leads. After the lead tips are unscrewed from the myocardium, gentle traction is applied to determine if the leads can easily be removed. If traction is unsuccessful, additional tools (both powered or mechanical sheaths) are used to complete the lead extraction29; the goal is to lyse and free the fibrotic attachments between parallel leads and between the leads and vessel wall or the myocardium. Once the lead is freed from the adhesions it can be removed safely.
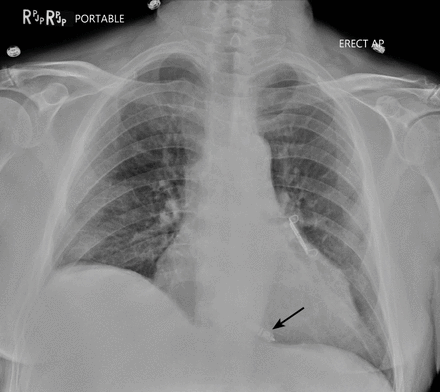
The incidence of major complications with lead extraction is low (1.8%), but the procedure can be life-threatening.30 Major complications include cardiac avulsion, vascular laceration, pericardial effusion, tamponade, hemothorax, valve injury, and death during the procedure.30 Risk factors for major complications with TLE include renal failure, low body mass index, and the presence of a defibrillator coil on the lead.30,31 In a large cohort of more than 3,000 patients requiring 6,000 TLE procedures at our tertiary care center, the incidence of catastrophic complications that required emergency cardiac surgery or vascular intervention was 0.8%.32 Many of these patients were rescued through emergency surgical repair of a venous laceration or cardiac perforation but still had an in-hospital mortality rate of 36%. Surgical lead extraction is usually performed if percutaneous lead extraction has failed, if epicardial leads are present, if large vegetations are attached to the leads, or if surgery is warranted for valvular involvement with endocarditis (Figure 2).14
In a patient with endocarditis after cardiac implantable electronic device placement, transthoracic echocardiography shows a large vegetation (V) near the right atrium (RA), right ventricle (RV), and across the tricuspid valve (TV). This required surgical extraction of the organized vegetation along with the device and leads.
REIMPLANTATION
The need for reimplantation after removal of an infected CIED should be thought about before the extraction. In general, extracting an infected CIED should be viewed as an opportunity to reassess the need for the device. Almost one-third of patients who undergo extraction of infected CIED do not require immediate reimplantation.2 This could be due to reversal of the initial indication, emergence of new clinical conditions, patient preference, or the lack of an absolute indication. If reimplantation is necessary, the new device is typically placed on the opposite side of the chest from the previously infected pocket site after blood cultures are negative for at least 72 hours.21
CIED INFECTION MORTALITY
Despite proper management with CIED removal supported by antibiotic therapy, CIED infection carries a high risk of death. The 30-day mortality is estimated to be between 5% and 6%.33 In a large case series of 412 CIED extractions, there were 19 in-hospital deaths. Of these 19 deaths, 2 were related to the extraction itself with the other 17 related to sepsis, multiorgan failure, stroke, renal failure, or heart failure.2 The 1-year mortality rate is also increased for this population; recent data show 1-year mortality rates of 8% to 17% despite device removal and antibiotic therapy.2,34,35 This increased mortality rate was also demonstrated in a large cohort of Medicare patients undergoing CIED procedures.36 Medicare patients with CIED infection had double the risk of death at 1 year compared with patients without infection.36
Risk factors for death at 1 year include worse baseline functional status, renal failure, and type of infection; eg, endovascular infection carries a risk of death 2 times higher than pocket infection.37
PREVENTION
Because CIED infection carries significant short-term and long-term mortality rates despite optimal management, the best strategy is prevention. Preventing CIED infection begins with the decision to implant a device with careful assessment of the indication, the timing of the procedure, and the patient’s clinical status. CIED procedures are performed under strict sterile surgical techniques with great attention to the incision and proper closure. Surgical data favor the use of chlorhexidine-alcohol solutions for skin preparation compared with povidone-iodine solutions to prevent both superficial and deep surgical wound infections.38 However, recent studies showed no significant difference between the 2 preparation methods in reducing rates of CIED infection.39,40 In individuals colonized with S aureus, the risk of CIED infection can be reduced using a body wash containing chlorhexidine and a nasal spray containing mupirocin.41,42
Preoperative antibiotics
The use of preoperative antibiotics has been shown to reduce the risk of infection.43 In a large prospective cohort of patients undergoing a de novo or secondary CIED procedure, the use of perioperative antibiotics was negatively associated with the risk of CIED infection.13 This was later confirmed by a double-blind randomized trial of 1,000 patients undergoing permanent pacemaker or ICD initial implantation or generator replacement. This study was stopped prematurely as the use of antibiotics was clearly associated with a lower risk of CIED infection.44 Therefore, prophylaxis with an antibiotic active against staphylococci before the incision is made is a class I indication to prevent infection.1
Currently, no data support giving prophylactic antibiotics after the procedure; however, the Prevention of Arrhythmia Device Infection Trial (PADIT) is currently comparing the risk of infection with conventional preoperative antibiotics vs a regimen of pre- and post-procedure antibiotics (clinicaltrial.gov: NCT01628666).
Hemostasis
Adequate hemostasis is critical, since the risk of CIED infection is 7 times greater with formation of a hematoma.45 Heparin products, especially low-molecular-weight heparin, should be avoided at the time of CIED implantation. In patients at high risk for thromboembolism who are on warfarin therapy, the continuation of warfarin is associated with a lower incidence of hematoma compared with bridging with heparin in patients undergoing CIED procedures.46
Therefore, if anticoagulation can be withheld, it is better to stop the anticoagulant before the procedure. When this is not possible or when it carries significant risk (eg, a patient with a mechanical mitral valve who needs a CIED implantation), it is better to maintain the patient on warfarin therapy with a therapeutic international normalized ratio rather than bridging with heparin products.
Antibacterial envelop and new devices
A new development in the prevention of CIED infection is the TYRX absorbable antibacterial envelope (Medtronic Inc.) (Figure 3), a multifilament knitted mesh coated with the antibiotics rifampin and minocycline, which are released in the device pocket over 7 days. The first-generation envelope was non-absorbable; the new product uses a fully bioabsorbable polymer that dissolves within 9 weeks. Data from nonrandomized studies using mainly the nonabsorbable version showed favorable outcomes in reducing the rate of CIED infections.47,48 The World-wide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT) is a large randomized clinical trial assessing the efficacy of the absorbable envelope in reducing CIED infection rates in patients undergoing CIED replacement or upgrade.49
The TYRX absorbable antibacterial envelope is a mesh coated with the antibiotics rifampin and minocycline, which elute off the mesh within approximately 7 days. The mesh is completely absorbed into the body in about 9 weeks.
The development of new cardiac devices carries the potential of reducing certain types of infection. The subcutaneous ICD is an entirely subcutaneous system with no endovascular component, and therefore it can prevent endovascular infection, especially in patients at high risk of infection (eg, patients on hemodialysis).50 On the other hand, the leadless pacemaker is a single-chamber pacemaker deployed percutaneously in the right ventricle without the need for a pocket, thereby eliminating the risk of pocket infection (Figure 4).51,52 Whether the risk of endovascular infection will be reduced is not yet known.
A leadless pacemaker in the right ventricle. The left atrial appendage exclusion clip is present.
CONCLUSION
CIED infection is a major complication that carries significant risk of morbidity and death. Early diagnosis and referral to a multidisciplinary treatment team is crucial to increasing the possibility of a cure. While device extraction has risks, it is nevertheless typically required for complete resolution of the infection. Large clinical trials are under way to address current knowledge gaps about CIED infection, including our understanding of the true incidence rate, risk factors, and efficacy of various implantation techniques. Future trends to minimize the risk of CIED infection include better screening, better diagnostic tools, new devices with fewer or no leads, longer battery life to minimize the need for additional procedures, and the use of supportive tools and products to minimize the risk of infection.
Footnotes
Dr. Lambert reported no financial interests or relationships that pose a potential conflict of interest with this article. Dr. Tarakji reported that he receives consulting/ advisory fees from Medtronic and AliveCor.
- Copyright © 2017 The Cleveland Clinic Foundation. All Rights Reserved.