ABSTRACT
Aneurysms of the renal artery and splenic artery are uncommon but clinically important, as they pose a risk of rupture with a high fatality rate. Indications for surgical or endovascular repair are based on aneurysm location and risk factors for rupture, such as aneurysm size, growth, and associated conditions, while medical management is also important. Regular surveillance with imaging is critical before and after intervention to guide treatment.
Renal and splenic artery aneurysms are often detected incidentally but can present acutely with dissection, rupture, or both, which are associated with high risk of death and morbidities.
Computed tomographic and magnetic resonance angiography are key to diagnosing and characterizing the aneurysm and the remaining vasculature, while ultrasonography helps in assessment and surveillance. Catheter angiography is the gold standard for diagnosis and allows the opportunity for intervention.
The individual’s risk for rupture or dissection determines the need for prophylactic intervention and is based on aneurysm size, location, growth, and other associated conditions and risk factors.
Management strategies include open and laparoscopic surgery and endovascular procedures. Regular imaging surveillance is critical after both diagnosis and interventions.
Although aneurysms of the abdominal and thoracic aorta are more common, visceral aneurysms such as those of the renal artery and splenic artery can also form.
In a previous article,1 we discussed the optimal surveillance strategies and treatment for aneurysm of the thoracic aorta. Here, we address the diagnosis, surveillance, and treatment of renal artery and splenic artery aneurysms.
RENAL ARTERY ANEURYSM
What is the clinical importance of renal artery aneurysm?
Renal artery aneurysms are rare, found in about 0.1% of the population in autopsy studies, although significantly more in cross-sectional imaging or angiography studies.2 They are categorized by location and morphology into 3 types (Figure 1), with important management implications:
Type 1—saccular aneurysm from the main renal artery or large segmental branch
Type 2—fusiform aneurysm
Type 3—intralobar artery aneurysm.3
Three types of renal artery aneurysms.
Renal artery aneurysms often present in the sixth decade. Up to 90% of patients have hypertension, and a minority have arterial aneurysms elsewhere.4 Most younger patients with renal artery aneurysms are women, and about two-thirds have fibromuscular dysplasia.
Symptoms are rare but can include hypertensive crisis, shock, hematuria, flank and abdominal pain, and urinary obstruction, with signs of a palpable abdominal mass and a renal bruit.2 The natural history is slow growth at 0.06 to 0.09 mm per year. The clinical concern is rupture, which occurs in 3% to 5%, with mortality rates near 10%, although these figures are lower than with other types of visceral aneurysms.2,5
How is renal artery aneurysm assessed?
Renal artery aneurysms are often diagnosed incidentally either during investigation of resistant hypertension or during angiography for another indication. They are detected more often now than in the past because of improvements in imaging.2
Blood pressure and renal function should be routinely assessed. Accurate measurement is important, as size is directly associated with risk of rupture and need for intervention. Circumferential calcification may be protective.2
Duplex ultrasonography is the least expensive imaging modality. Further, it does not expose the patient to radiation, and it can show a dilated vessel with turbulent flow. On the other hand, it has the lowest sensitivity and specificity, and its accuracy depends on operator experience and the patient’s body habitus.6
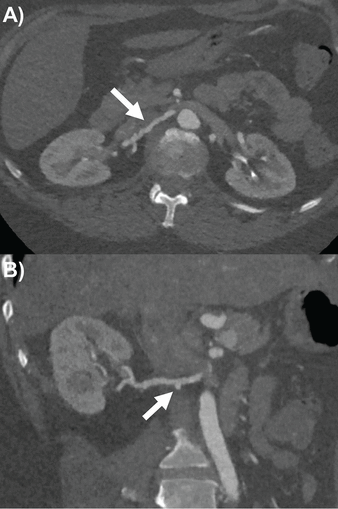
Multidetector computed tomographic angiography (CTA) is the most commonly used modality for assessing renal artery aneurysms. Its superior resolution can characterize anatomy using multiplanar reconstructions and volume-rendered imaging (Figure 2). On the negative side, it entails the use of nephrotoxic contrast media and radiation exposure.7
Right renal artery ectasia and beading with focal aneurysm (arrows) by computed tomography angiography in patient with fibromuscular dysplasia on (A) axial slice and (B) double oblique multiplanar reconstruction.
Magnetic resonance angiography (MRA) has become an alternative to CTA, with comparable accuracy and without radiation, but it is less available and costs more.6,7
Catheter angiography remains the gold standard invasive option for diagnosing renal artery aneurysm, allowing assessment of proximal and distal aneurysms, and providing the opportunity for percutaneous interventions.2
If a renal artery aneurysm is found but does not meet the criteria for intervention (see below), repeat imaging at 1, 6, and 12 months and then annually has been recommended by some experts, although guidelines are lacking.2 Ultrasonography can be used for surveillance, and abnormalities can be confirmed by any of the other 3 modalities. Annual surveillance should also be considered after surgery at least initially, and long-term after endovascular therapy.2,8
How should renal artery aneurysm be treated?
There are no guidelines for treatment of renal artery aneurysm, and if the aneurysm has not ruptured, indications for prophylactic surgery are based on rupture risk.2 These include any of the following4:
Large size (> 2 cm)
Symptoms
Refractory hypertension with significant renal artery stenosis or thromboembolism
Childbearing age, for women.
The two main intervention options are surgical and endovascular repair. Acute rupture calls for emergency surgical repair of the aneurysm with renal artery reconstruction or with nephrectomy if the kidneys are not salvageable. For elective management, surgical primary repair or endovascular repair with stents or coil occlusions can be performed for type 1 aneurysm; surgical reconstruction with a vein graft or aortorenal bypass graft typically for type 2 aneurysm; and embolization coils for type 3 aneurysm, with renal preservation whenever possible.2,9
Mortality rates during elective interventions have been less than 5% in recent studies, but up to 50% in pregnancy. Surgical complications include occlusion of the renal artery, branch, or graft; renal ischemia; and cardiac events. Endovascular complications include failed procedure (< 10%), thrombosis, embolization, and postembolization syndrome.2,10 “Postembolization syndrome” refers to a constellation of symptoms including abdominal pain, fever, and at times ileus and pancreatitis that occur in up to 30% of patients after abdominal visceral arterial embolization.11
Overall, outcomes have improved significantly over time with earlier detection and better planning and interventional techniques.2,4
SPLENIC ARTERY ANEURYSM
What is the clinical importance of splenic artery aneurysm?
The prevalence of splenic artery aneurysm is 0.04% to 0.10% at arteriography and autopsy. It is often found incidentally, but accounts for about 60% of visceral arterial aneurysms.12
The main risk factors or causes include portal hypertension, liver transplant, pregnancy, pancreatitis, atherosclerosis, hypertension, connective tissue disease (eg, Marfan syndrome), vasculitis, endocarditis, fibromuscular dysplasia, trauma, congenital anomalies, infection, older age, and female sex (especially multiparous women).11,12
Symptoms occur in up to 20% of cases and include upper abdominal pain that can radiate to the shoulder, nausea, vomiting, anorexia, and gastrointestinal bleeding. Rupture presents with acute abdomen, peritoneal bleeding, and shock.12,13 The risk of rupture is 2% to 10%, with a mortality rate of 25%, and both figures are markedly higher in pregnancy.12,14
How should splenic artery aneurysm be evaluated?
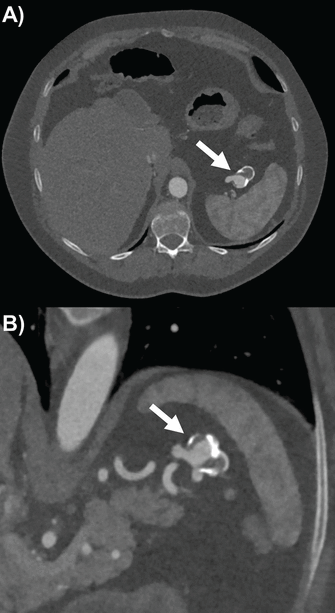
Abdominal radiographs rarely detect splenic artery aneurysm. Ultrasonography has some utility as an accessible, low-cost, and radiation-free tool, with varying sensitivity for splenic artery aneurysm depending on the operator’s experience and the patient’s body habitus.12 Therefore, CTA (Figure 3) and MRA are again the preferred imaging modalities for diagnosing splenic artery aneurysms. Both can provide 3-dimensional reconstruction for accurately assessing the aneurysm’s dimensions, the neighboring vasculature, and other abdominal pathologies that could contribute to its cause.12,13 Endoscopic ultrasonography can also assist in the diagnosis and differentiate splenic artery aneurysm from nearby splenic and pancreatic pathology, such as pancreatic pseudocyst.13,15 Catheter angiography, although invasive, continues to be the gold standard for characterizing the aneurysm’s location, size, and extent.12,13
Splenic artery aneurysm (arrows) partially calcified and thrombosed by computed tomography angiography on (A) axial slice and (B) double oblique multiplanar reconstruction showing proximal and distal end of vessel.
If intervention is not planned, surveillance is recommended at 6 months after diagnosis and then annually. Ultrasonography is suitable for surveillance if it can adequately characterize the aneurysm. CTA is also an option.12
How should splenic artery aneurysm be managed?
In the absence of guidelines, the main recommended indications for intervention of splenic artery aneurysm are rupture, aneurysm size larger than 2 or 2.5 cm, growth of the aneurysm by 3 to 5 mm or more during surveillance regardless of initial size, symptoms, women of childbearing age, portal hypertension, and planned liver transplant.11,12
The treatment options again are open surgery (mandatory in the setting of rupture), endovascular procedures, and laparoscopic surgery.
Open surgical procedures include ligation of the splenic artery or aneurysm and aneurysmectomy with or without splenectomy. Mortality rates are around 1%.
Endovascular options include transcatheter embolization, covered stent-graft insertion, and coil or thrombin injection or both, and these have now become first-line for elective management when anatomically feasible because of lower mortality and complication rates than with open surgery.16
Minimally invasive laparoscopic surgery has become an alternative. It is associated with less pain and shorter length of stay compared with open surgery, and it allows splenectomy and distal pancreatectomy to be performed if needed.13
Potential complications of intervention include postembolization syndrome, splenic infarction or abscess, and pancreatitis. Therefore, follow-up imaging with CTA or ultrasonography is recommended.12
Footnotes
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.