ABSTRACT
Most patients with cancer experience pain at some point in the disease course due to the disease itself or its treatment, or both. Pain management can involve pharmacologic (nonopioid medications, adjuvants, and opioids) and nonpharmacologic (radiation therapy, interventional procedures) therapies. This article provides a treatment approach to reduce pain for patients with cancer and improve their quality of life.
Cancer pain affects patients throughout the disease trajectory.
The typical pharmacologic regimen for treating patients with cancer pain consists of an assortment of nonopioid analgesics, adjuvant pain medications, and opioids.
Early consideration of radiation therapy and various interventional pain management procedures can optimize pain control and preclude escalation of opioids.
New or worsening pain in patients with a history of cancer requires thorough assessment for cancer recurrence or progression.
Cancer-related pain, resulting from the disease itself, its treatment, or both, is one of the disease’s most agonizing symptoms, severely diminishing quality of life. A review of 52 studies published from 2005 to 2014, with 32,261 patients, concluded that 50.7% of patients with cancer experienced pain.1 In those who completed curative treatment, the prevalence of pain was 39.3%, in those receiving anticancer therapy it was 55%, and in those with advanced, metastatic or terminal disease it was 66.4%.
Because cancer-related pain occurs throughout the course of the disease, primary care providers are likely to be called on to manage cancer pain, either in the outpatient or inpatient setting. Whether the provider is caring for a patient around the time of diagnosis, during treatment, at the terminal phase, or in survivorship, effective treatment of cancer pain helps patients achieve optimal quality of life. Knowledge of therapeutic approaches and both pharmacologic and nonpharmacologic alternatives may also assist clinicians in treating patients before partnering with specialists, such as those in oncology, palliative medicine, and pain management.
In an earlier article in this journal, Induru and Lagman2 stressed that effectively managing cancer pain can lead to overall improvement in patient satisfaction and quality of life. They explored the use of drugs such as opioids and adjuvant pain medications and nonpharmacologic measures such as acupuncture, massage therapy, and music therapy. This article builds on the previous review and features novel drugs and other, nonpharmacologic interventions available for patients with cancer. It also examines pain management in cancer survivors.
WHAT ARE THE KEY PRINCIPLES IN MANAGING CANCER PAIN?
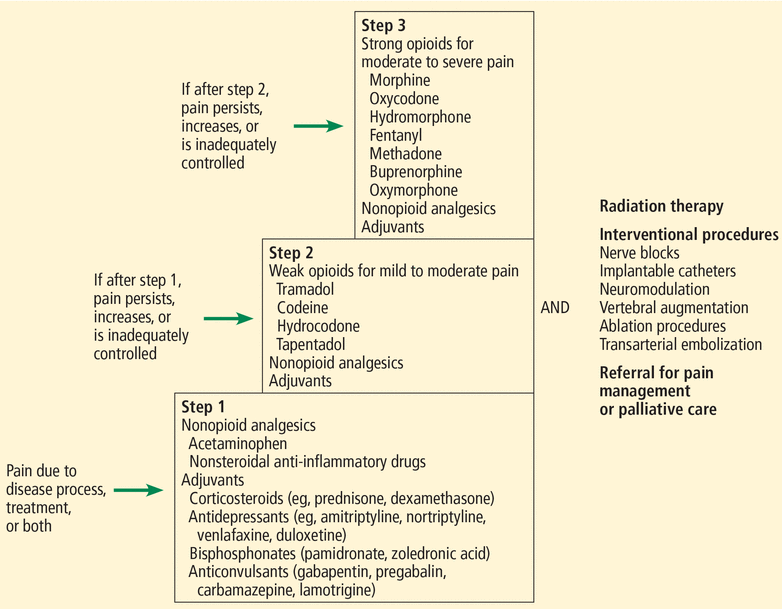
Cancer pain management often involves both drugs and procedures (Figure 1). The most commonly used framework that clinicians can employ in deciding which analgesic drugs to use is the World Health Organization (WHO) analgesic ladder.2,3
Our approach to managing cancer pain, based on the World Health Organization analgesic ladder.
At the base, or step 1, of the 3-step ladder are nonopioid analgesics (eg, acetaminophen, nonsteroidal anti-inflammatory drugs) and adjuvants, which are used for mild pain. Adjuvants are drugs that are primarily indicated for conditions other than pain but that possess analgesic properties; they include corticosteroids (eg, prednisone, dexamethasone), antidepressants (eg, amitriptyline, nortriptyline, venlafaxine), bisphosphonates (eg, pamidronate, zoledronic acid), and anticonvulsants (eg, gabapentin, pregabalin, carbamazepine, lamotrigine).
If pain persists or increases, weak opioids for mild to moderate pain (eg, tramadol, codeine, hydrocodone) are added in step 2. If pain remains uncontrolled, strong opioids for moderate to severe pain (eg, morphine, oxycodone, hydromorphone, fentanyl, methadone, buprenorphine) are used in step 3.
At any stage, when cancer pain persists, escalates, or remains inadequately controlled, clinicians should consider specific nonpharmacologic interventions, which will be discussed below. Providers of ancillary services—nursing, social work, physical and occupational therapy, spiritual care—may need to be called in. Similarly, specialists from fields such as radiation oncology, palliative medicine, pain management, anesthesiology, interventional radiology, surgery, and orthopedics may be essential in optimizing pain control. We recommend collaborating with specialists in either palliative medicine or pain management when step 1 of the WHO analgesic ladder fails to provide ample relief, especially for providers who are uncomfortable prescribing opioids.
WHAT ARE SOME OF THE NONOPIOID MEDICATIONS USED FOR CANCER PAIN?
Duloxetine, an antidepressant
Duloxetine, a serotonin-norepinephrine reuptake inhibitor, is primarily used to treat depression and anxiety, but it is increasingly finding a place in the treatment of diabetic neuropathy, fibromyalgia, chronic back pain, and osteoarthritic pain.4–6 Recent studies suggest that duloxetine, alone or in combination with opioids and gabapentinoids (eg, gabapentin, pregabalin), also offers benefit in 2 cancer-related pain conditions, chemotherapy-induced peripheral neuropathy and cancer-related neuropathic pain.7,8
The dosing used in studies ranged from 20 to 60 mg per day. Common adverse effects are nausea, fatigue, and both insomnia and somnolence.
Cannabinoids are not recommended
The 2 most prominent and abundant cannabinoids—compounds derived from the Cannabis sativa plant—are tetrahydrocannabinol (THC) and cannabidiol (CBD). Preparations of cannabinoids that patients can access generally come in 3 forms:
THC-dominant, eg, oral dronabinol and nabilone
Balanced THC-CBD, such as oromucosal nabiximols
CBD-dominant, such as oral CBD oil solution.9
Medical cannabis or marijuana preparations of all of these 3 forms are available and can be taken by various routes, such as by mouth, inhaled, or topical application.
Cannabinoids have been studied for the treatment of several cancer symptoms, notably pain, anorexia, nausea, and dysgeusia. Five randomized controlled trials from 2012 to 2018 were part of a systematic review of cannabis-based medicines for cancer pain published in 2019.10 The review concluded that neither balanced THC-CBD nor THC-dominant preparations differ from placebo in reducing pain. Adverse effects of cannabinoids noted in studies include dizziness, dry mouth, nausea and vomiting, somnolence, confusion, and memory impairment.9,10 Of note, nabiximols, the cannabinoid most studied for treating cancer pain, is not currently available in the United States.
The lack of quality evidence, access issues, and worrisome side effects preclude the use of cannabinoids for cancer pain at this time.
Acetaminophen
Acetaminophen (also called paracetamol) is a nonopioid medication used in step 1 of the WHO approach to managing cancer pain. Widely available in various formulations and brands, it is a popular analgesic, formulated by itself or combined with other drugs.
With regard to cancer pain, a Cochrane systematic review in 2017 concluded that adding acetaminophen to a daily regimen of 60 mg or more of oral morphine results in no additional benefit in terms of pain relief, quality of life, or patient satisfaction or preference.11 The review was based on 3 randomized, placebo-controlled trials with 122 participants, in which the daily acetaminophen dose ranged from 3,000 mg to 4,000 mg.12–14 The reviewers also noted that they could find no study that used acetaminophen alone for cancer pain.
Based on these findings, acetaminophen may not be of benefit when used in step 3 of the WHO analgesic ladder.
WHAT ARE SOME OF THE NEW OPIOID MEDICATIONS FOR CANCER PAIN?
Tapentadol, a mu agonist and norepinephrine reuptake inhibitor
Tapentadol has a unique synergistic mechanism of action, functioning as both a weak mu-opioid agonist and a norepinephrine reuptake inhibitor, making it the first in a new drug class.15–17 While tapentadol has 50 times less affinity for the mu-opioid receptor and relatively moderate norepinephrine reuptake inhibitor activity, the synergy of these mechanisms generates a degree of potency comparable to that of morphine.17
This unique mechanism results in potential benefits. The drug causes fewer adverse effects than other opioids, especially gastrointestinal problems such as nausea, vomiting, and constipation. The time to development of tolerance is longer than with morphine, and the likelihood of abuse may be lower.15,16 Tapentadol can also be helpful in treating neuropathic pain, with a similar mechanism as tricyclic antidepressants and serotonin-nor-epinephrine reuptake inhibitors.
Tapentadol is available in both immediate-and extended-release forms. There is currently no generic version available in the United States, and the drug may be prohibitively expensive or require prior insurance authorization.
Oxymorphone, a semisynthetic mu agonist
Oxymorphone is a semisynthetic mu-opioid agonist that is about twice as strong as oxycodone and 3 times as strong as oral morphine in relieving pain.18 It has been shown to be clinically comparable to oxycodone, and it caused less respiratory depression in 1 study.18 It is available in both immediate- and extended-release formulations.
The drug is predominantly metabolized in the liver, and, therefore, its use is relatively contraindicated in patients who have moderate to severe liver failure.15 Its elimination in renal failure is prolonged; hence, a longer dosing interval is recommended.
Oxymorphone has been shown to be effective and well tolerated for managing cancer pain,19,20 and can be considered for patients for whom other strong opioids such as morphine, oxycodone, and fentanyl have failed or who could not tolerate these drugs. Of note, oxymorphone, either in immediate- or extended-release form, generally costs more in the United States than morphine or oxycodone.
New fentanyl formulations
While intravenous and transdermal fentanyl preparations are used fairly often, a number of newer formulations are available. Transmucosal fentanyl products have been available in the form of buccal tablets, films, and intranasal sprays for a number of years, but are restricted in their use to opioid-tolerant patients (ie, those taking daily doses of at least 60 mg oral morphine or its equivalent for at least 1 week),21 and are relatively expensive, limiting their use.
The advantages of these forms of fentanyl are rapid onset (within 10–15 minutes) and short duration of action, making them particularly beneficial in treating episodes of unpredictable breakthrough pain.21,22 Available dosages, however, do not correspond with those of other opioids, and even doses of different fentanyl formulations given by the same route are not equivalent. Each preparation must be started at the lowest available dose and titrated up to effect when starting or changing formulations.
WHAT IS THE ROLE OF RADIATION THERAPY IN MANAGING CANCER PAIN?
Radiation therapy, or radiotherapy, has various roles in treating cancer; it is given with intent to cure the disease, arrest tumor growth, or control symptoms. As a nonpharmacologic analgesic, it is effective and time-efficient.23 In particular, it should be strongly considered for patients suffering from painful bone metastases.
Radiation therapy generally is of 2 types, external beam and stereotactic. External beam radiation therapy, the conventional type, uses a fixed source of radiation directed toward cancerous tissue in the body. Stereotactic therapy, on the other hand, uses a moving source that targets the tumor from different angles, thereby limiting damage to nearby normal tissue. This type is typically selected for small or mediumsized tumors.
In either type, treatment can be delivered in single or multiple doses or fractions. A single fraction has been shown to be as efficacious as multiple fractions for alleviating pain from bone metastases.24 Of note, more patients who undergo single-fraction therapy subsequently need repeat radiation therapy to the same site compared with those who receive multiple fractions up front.25 Single-fraction therapy has the advantage of being cost-effective and convenient, especially for patients with limited life expectancy.
Of note, a transient “pain flare” can occur with radiotherapy. In a study of patients who underwent single- or multiple-fraction radiation therapy for symptomatic bone metastases, the overall incidence of pain flare within 10 days of completion was 40%.26 The corticosteroid dexamethasone, given immediately before single-fraction therapy and daily for 4 days after, has proven to mitigate pain flares.27
WHAT INTERVENTIONAL PROCEDURES ARE AVAILABLE FOR CANCER PAIN?
Some patients with cancer experience refractory pain, defined as failure of conventional oral pharmacologic agents and tumor-directed radiation therapy to control pain or such treatment causing intolerable side effects. In this situation, interventional treatments should be considered (Table 1). However, evidence of efficacy is lacking. Most data on outcomes are based on case reports and case series, given challenges in methodology such as accrual of adequate sample sizes for test populations and control groups.28
Interventional procedures for cancer pain management
Nerve blocks
Cancer pain can affect nearly any anatomic site and may need local control using nerve or neurolytic blocks, which can be achieved by chemicals (phenol or ethanol), radiofrequency (thermal) ablation, or surgery. Nerve blocks can be performed in a peripheral nerve, plexus nerve, or central neuraxial site. In theory, any peripheral nerve can be blocked, but technical difficulties (eg, scar tissue, swelling) may preclude the procedure, and outcomes cannot be predicted due to a dearth of evidence.29 Examples of localized nerve blocks include paravertebral blocks for patients undergoing breast surgery30 and interscalene blocks for surgical repair of pathologic fractures.31
Celiac plexus blocks are often used and are well studied in treating abdominal pain from pancreaticobiliary cancers. They have been shown to lower pain scores and decrease opioid use.32–35 Ultrasonography-guided endoscopic celiac plexus blocks have also been performed. Though mostly based on case reports and low-quality studies with small sample sizes, positive outcomes have been described.36,37
Blocks to the superior hypogastric plexus for pelvic pain from gynecologic and urologic malignancies and to the ganglion impar (ganglion of Walther) for perineal pain secondary to anorectal tumors have been shown to resolve pain.38,39 Injection can be done into the intrathecal space to achieve segmental pain control without affecting motor function.40
Implantable catheters and neuromodulation
For intractable tumor-related abdominal pain, neuropathic pain in extremities, or somatic low-back pain, another method of achieving central neuraxial analgesia is to use a percutaneous or implanted catheter to deliver opioids, local anesthetics, and adjuvant analgesics into the epidural or intrathecal space.28 The dose is smaller than a systemic dose, and this route would likely benefit an individual having severe adverse effects from systemic opioid therapy.41,42
A randomized controlled trial in 202 patients with advanced cancer compared medical management alone vs intrathecal delivery plus medical management. The latter was associated with lower pain scores, fewer side effects, and increased survival.43,44
Neuromodulation is the delivery of electricity to peripheral nerves, spinal cord, and brain. Spinal cord stimulation is commonly used to treat neuropathic pain from failed back syndromes, ischemic limbs, and complex regional pain syndromes, even though there is a paucity of evidence.45 It has been applied to cancer pain and shown to decrease pain scores and opioid use, based on case reports.46–48 Reports of dorsal root ganglion stimulation may help pain from surgery, complex regional pain syndrome and phantom pain, and may be considered for refractory neuropathic cancer pain.49
Vertebral augmentation
Vertebral augmentation involves injecting polymethyl methacrylate, a cement, directly into the vertebral body (vertebroplasty) or through a balloon (kyphoplasty). Patients with vertebral compression fractures from spinal metastases may benefit from either procedure.
Kyphoplasty was shown to improve pain scores, decrease opioid use, and improve quality of life compared with medical management alone in a randomized controlled trial in 134 patients with cancer.50 Several studies showed improved pain scores and physical function after this procedure in patients with painful, cancer-related vertebral fractures.51–53
Ablation procedures
Imaging-guided tumor ablation involves destruction of bone or soft tissue using radio frequency energy, cold (cryoablation), microwave energy, or magnetic resonance imaging-guided focused ultrasound.
Radiofrequency ablation has been the most used. Patients experienced reduced pain scores and improved mood after the procedure.54–56 Combined radiofrequency ablation and cementoplasty, in which cement is injected into bone for stabilization, have been shown to improve outcomes, as the latter provides structural stability to bone destroyed by the ablation.57–59
Patients treated with cryoablation experienced improved pain scores, decreased opioid use, and durable effects (ie, lasting 24 weeks or more).60–63
A prospective 1-year study of computed tomography-guided microwave ablation of bone metastases and soft-tissue sarcomas demonstrated a success rate (defined as ≥ 80% tumor necrosis) of 80% at 1 month and 63% at 12 months.64 Combined with cementoplasty, microwave ablation decreased pain scores and improved ambulation in a retrospective study of 35 cancer patients with high risk of fracture.65
Magnetic resonance imaging-guided focused ultrasound provides more defined tumor margins for a more accurate target ablation.66 Case series from both single centers and multiple centers showed improved pain scores and decreased opioid analgesic use.67,68
Transarterial embolization
Often, before orthopedic surgery, an occlusive material is injected intra-arterially to prevent perioperative bleeding from potentially bloody bone metastases.69 This practice, called transarterial embolization, has provided pain relief for metastatic bone disease in several case series.70–72
HOW IS PAIN MANAGED IN CANCER SURVIVORS?
Over the years, effective treatments and innovations have yielded remarkably improved life expectancy and cure rates for patients with most types of cancer. Unfortunately, more than a third of survivors continue to suffer from cancer pain.1
Chronic pain in these patients can be caused by any of the 3 primary anticancer treatment approaches—chemotherapy, radiation therapy, or surgery (Table 2).73,74 Many patients undergo a combination of these treatments, resulting in complex pain. Other causes of chronic pain include lymphedema, osteoporosis leading to pathologic fractures, and adjuvant drugs (eg, aromatase inhibitors, used to treat breast cancer, which cause myalgia and arthralgia).
Causes of chronic pain associated with cancer treatment
Pain management in cancer survivors mirrors the interdisciplinary and multimodal approach for treating pain in patients undergoing active treatment. Rather than an ongoing search for a cure of the pain, preserving function and adopting coping strategies become the focus for survivors.73
In managing these patients, it is crucial to thoroughly assess new or worsening pain, especially when accompanied by such symptoms as unexplained weight loss, unusual fatigue, altered bladder or bowel function, persistent cough, focal numbness or weakness, or an enlarging mass.74 This allows prompt diagnosis of cancer recurrence or progression.
The WHO pain relief ladder remains the framework for starting analgesics, with close attention to employing adjuvant medications to control neuropathic pain. Opioids need to be used carefully, and with the eventual goal of weaning. Nonpharmacologic interventions, such as nerve blocks, may be indicated for patients with refractory surgery-related pain. Patients who acquire the tendency to catastrophize or exaggerate their pain may benefit from psychosocial support provided by a social worker, psychologist, or spiritual counselor. Referral for physical therapy, occupational therapy, or both may be necessary to improve functional status in the face of chronic pain.
A COMPREHENSIVE APPROACH TO A COMPLEX PROBLEM
Managing cancer pain across its disease trajectory is complex. A comprehensive, interdisciplinary, and multimodal approach combining pharmacologic and nonpharmacologic interventions provided by various disciplines and medical specialties is vital.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Acknowledgments
We would like to thank Beth Faiman, PhD, MSN, for reviewing our article and sharing her valuable comments.
- Copyright © 2021 The Cleveland Clinic Foundation. All Rights Reserved.