A 64-year-old woman who had recently immigrated to the United States from Vietnam came to the emergency department because of a painful mass in the left side of her jaw, which she had first noticed 3 weeks earlier. Because the pain kept getting worse and the mass kept getting bigger, she had gone to her dentist, who gave her amoxicillin, which did not help. She also reported headache, severe trismus (“lockjaw”), odynophagia (painful swallowing), night sweats, and unintentional loss of 6 kg (13 lb) over several months. She said she had experienced no recent trauma or fever, had never used tobacco, alcohol, or illicit drugs, and had never been seriously ill before.
INITIAL EVALUATION AND MANAGEMENT
In the emergency department, her temperature was 98.1°F (36.7°C), pulse 74 beats per minute, blood pressure 132/66 mm Hg, respiratory rate 18 breaths per minute, and oxygen saturation 99% while breathing room air.
On examination, she had a large, tender mass at the left mandibular angle and marked tenderness to palpation in the back of the left side of her neck. Her teeth could not be well visualized due to trismus. She also had swollen submandibular lymph nodes on the left side, ulceration and erythema along the left retromolar trigone, and numbness on the left side of her face in the area supplied by the mandibular nerve. The rest of the physical examination was unremarkable.
Laboratory test results at presentation were the following:
White blood cell count 3.1 × 109/L (reference range 4.5–11.0 × 109/L)
Absolute neutrophil count 1.6 × 109/L (1.8–7.7 × 109/L)
Absolute lymphocyte count 0.7 × 109/L (1.0–4.8 × 109/L)
Absolute monocyte count 0.6 × 109/L (0.2–0.4 × 109/L)
Absolute immature mononuclear cell count 0.1 × 109/L (0.0 × 109/L)
Hemoglobin 11.9 g/dL (12–16 g/dL)
Hematocrit 34.0% (36%–46%)
Mean corpuscular volume 101.5 fL (80–100 fL)
Platelet count 705 × 109/L (130–400 × 109/L)
Peripheral smear: Macrocytes present. Leukocytes were decreased with rare blast cells. Platelets were increased, with scattered large forms.
Computed tomography (CT) of the neck revealed a permeating mass in the ramus of the left mandible with a large soft-tissue component, measuring 6.2 cm in the anteroposterior dimension, 5.8 cm in the transverse dimension, and 6.3 cm in the craniocaudal dimension (Figure 1). Inseparable from the left muscles of mastication, the mass displaced parapharyngeal fat medially and extended posteriorly, abutting the left parotid gland.
Computed tomography of the neck on presentation was notable for a mass in the ramus of the left mandible with a large soft-tissue component (circle) within the left masticator space.
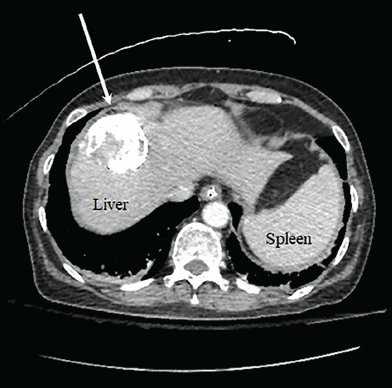
CT of the chest showed several hypodense lesions in the liver. One lesion with mixed hyperdense and hypodense material measured 4.6 by 5.0 cm.
DIFFERENTIAL DIAGNOSIS
1. In light of these findings and the uniquely high incidence of specific cancers in the Southeast Asian population, which of the following is the least likely cause of this patient’s mandibular mass?
Nasopharyngeal carcinoma
Odontoma (a benign tumor of dental tissue)
Metastatic lung cancer
Squamous cell carcinoma of the oral cavity
Mandibular lesions are often classified by the tissue of origin (odontogenic, nonodontogenic), and CT is crucial in guiding the initial diagnostic workup. Odontogenic lesions usually surround a component of the tooth. Additional features such as location within the mandible, cystic vs solid appearance, border contour with lytic or sclerotic features, and compression of surrounding tissues help to delineate the etiology of these lesions.1,2 Other clinical information such as the patient’s age, comorbidities, and risk factors may help to narrow the diagnosis. Nevertheless, tissue biopsy is often required to obtain a definitive pathologic diagnosis.
Odontomas are the most common odontogenic tumor of the mandible and are usually diagnosed during the second decade of life. Nearly 50% of these tumors are associated with an impacted tooth, and they often resemble normal teeth, as the lesion consists of various odontogenic components including dentin and enamel.2 This diagnosis was unlikely in our patient, given the lack of association with teeth and the large soft-tissue component seen on CT of her neck.
Mandibular masses related to primary head and neck cancer are typically the result of direct invasion. In view of our patient’s ethnic background, we considered primary nasopharyngeal carcinoma and squamous cell carcinoma of the oral cavity as potential diagnoses. While nasopharyngeal carcinoma is rare in the United States and Western Europe, it is endemic in Southern China and has intermediate incidence in Southeast Asian populations.3 It frequently originates from the posterolateral recess of the pharyngeal wall and presents as a cervical mass in the apex of the posterior cervical triangle.4 Additionally, more than half of oral cancers occur in Asia, with 11% of these occurring in Southeast Asia.5 Typically, these cancers metastasize to cervical lymph nodes and the lungs, although spread to the liver and bone has also been described.6
Rarely, mandibular masses can be a sign of a widespread metastatic disease process, most commonly breast, lung, or renal cell cancer.7 Of these, lung cancer is the most common cancer type and a leading cause of death in South, East, and Southeast Asia.8,9 Moreover, 20% to 40% of patients with lung cancer have bone metastases at the time of presentation.10,11
CASE CONTINUED: A REVELATION FROM THE PATIENT’S FAMILY
To identify the etiology of the patient’s mandibular mass, we performed fine-needle aspiration of the lesion. When we discussed the initial findings with the patient and her family, her children revealed that she had undergone treatment for liver cancer in Vietnam but that they had not disclosed the diagnosis to her. Given this additional information, we pursued further workup to evaluate for potential metastatic and recurrent hepatocellular carcinoma.
The results of further laboratory testing and imaging were as follows:
Carcinoembryonic antigen < 0.5 ng/mL (reference range < 5.0 ng/mL)
Alpha-fetoprotein level 22,728 ng/mL (< 8.8 ng/mL).
Additional radiographic imaging
CT with contrast of the abdomen and pelvis showed a 4.7-cm area within the dome of the right hepatic lobe containing hyperdense material with hypodense areas, likely related to prior transarterial chemoembolization treatment (Figure 2). Magnetic resonance imaging (MRI) (Figure 3) showed a 1.7-cm hypodense lesion within hepatic segment 6, in addition to scattered low-attenuation lesions smaller than 1 cm in the inferior right hepatic lobe and a 1.6-cm hypodense lesion within the spleen, which were worrisome for metastatic and residual disease.
Computed tomography of the abdomen demonstrating a 4.7-cm lesion in the dome of the right hepatic lobe (arrow) containing hyperdense material with hypodense areas, likely related to earlier transarterial chemoembolization treatment.
Magnetic resonance imaging of the abdomen reveals a 1.7-cm lesion in hepatic segment 6 (left arrow) and a 1.6-cm hypoenhancing lesion within the inferior pole of the spleen (right arrow).
Multiphase abdominal MRI showed 2 lesions in 2 different hepatic lobes, categorized as LR-5 (definitely hepatocellular carcinoma) by the diagnostic criteria of the Liver Imaging Reporting and Data System (LI-RADS).
Liver biopsy confirms the diagnosis
To obtain pathologic correlation, liver biopsy was performed. Hepatocyte paraffin-1, glypican-3, and arginase-1 were positive on immunohistochemical staining, confirming hepatocellular carcinoma. Results of the fine-needle aspiration of the mandible showed a similar patchy pattern of arginase-1 staining and shared histopathologic features with the liver sample, consistent with metastatic disease.
PATHOGENESIS AND DIAGNOSIS OF HEPATOCELLULAR CARCINOMA
Results of serologic tests obtained as part of an infectious disease workup initiated earlier during the hospitalization showed:
Human immunodeficiency virus antibody negative
Epstein-Barr virus viral load < 750 copies/mL (normal < 750)
Hepatitis C antibody negative
Hepatitis B surface antibody (anti-HBs) negative
Hepatitis B surface antigen (HBsAg) positive
Total hepatitis B core antibody (anti-HBc) positive.
2. The results of our patient’s hepatitis B serologic tests (anti-HBs negative, HBsAg positive, and anti-HBc positive) are most consistent with which of the following?
Acute infection
Chronic infection
Acute or chronic infection
Recovery from an acute infection
Hepatitis B serologic testing measures the levels and titers of several hepatitis B virus-specific antigens and antibodies, which are used to determine the phase of infection (Table 1).
Interpretation of hepatitis B serologic markers
At 4 to 10 weeks after exposure to the virus, HBsAg becomes detectable in the blood, followed by immunoglobulin M (IgM) anticore antibodies.12 Accordingly, in the acute phase of the infection, HBsAg, total anti-HBc, and IgM anti-HBc are positive. A resolving infection is characterized by the disappearance of HBsAg and the subsequent emergence of anti-HBs within 4 to 6 months. Of note, as the appearance of anti-HBs may be delayed after HBsAg clearance, sometimes anti-HBc is the only serologic marker of hepatitis B virus infection. Since HBsAg is the antigen used to generate an immune response to the hepatitis B vaccine, the presence of anti-HBs reflects not only recovery and natural immunity but also immunity as a result of vaccination.12
While most individuals will clear the hepatitis B virus, an estimated 5% of immunocompetent adults progress to chronic infection.13 In persistent infection, HBsAg will remain but at lower titers than during primary infection. As total anti-HBc indicative of previous or ongoing infection will persist for life, the presence of IgM antibodies to the hepatitis B core antigen can help to delineate acute from chronic infection.12 Therefore, our patient’s initial laboratory results were consistent with either acute or chronic infection. As IgM anti-HBc was negative, we concluded she had chronic infection.
Other serologic markers of interest are hepatitis B e antigen (HBeAg) and antibody (anti-HBe). Like the surface antigen, the e antigen indicates active viral replication, appearing during an acute infection and remaining only if the primary infection does not clear.12 The continued presence of HBeAg and delayed seroconversion to anti-HBe reflect high levels of hepatitis B virus DNA with chronic infection. HBeAg levels help with determining when to initiate hepatitis B virus-directed treatment, and high HBeAg levels are a significant risk factor for the development of hepatocellular carcinoma.14 Our patient was found to be HBeAg-positive and anti-HBe-negative.
HEPATOCELLULAR CARCINOMA: EPIDEMIOLOGY, RISK FACTORS, AND DIAGNOSIS
Liver cancer is the fourth most common cause of cancer-related death worldwide and is the most common cancer type in Southeast Asia, with hepatocellular carcinoma accounting for 75% to 85% of cases.9,15
The incidence of hepatocellular carcinoma varies by geographic location, with 72% of cases occurring in Asia vs 5% in North America.16 This variation is likely due to differences in exposure to risk factors, particularly hepatitis viruses. Worldwide, hepatitis B is the main cause of hepatocellular carcinoma, especially in Asia and sub-Saharan Africa due to low vaccination rates. In Western countries, hepatitis C virus is the leading cause of hepatocellular carcinoma. While cirrhosis of any etiology increases the risk of hepatocellular carcinoma, hepatitis B virus has a direct oncogenic effect regardless of the presence of underlying liver fibrosis, as seen in this patient.17 In patients with hepatitis C, hepatocellular carcinoma occurs commonly in those who have advanced-stage fibrosis.
Other risk factors for hepatocellular carcinoma include alcohol use, tobacco exposure, nonalcoholic steatohepatitis (NASH), and co-infection with human immunodeficiency virus. Particularly in the United States, the high prevalence of NASH has raised concern. As the incidence of hepatocellular carcinoma has risen since 1999,18 NASH has been identified as the most common underlying risk factor for it and is present in 59% of cases.19 Like some hepatitis viruses, NASH may confer an increased risk of hepatocellular carcinoma independent of cirrhosis, and the pathogenesis of NASH-associated hepatocellular carcinoma involves immune and inflammatory responses, DNA damage, and oxidative stress.20
The diagnosis of hepatocellular carcinoma can be made with imaging alone, either with dynamic contrast-enhanced CT or MRI of the abdomen.15 Lesions are scored using LI-RADS to determine the likelihood of hepatocellular carcinoma, with categories LR-4 indicating probable and hepatocellular carcinoma and LR-5 indicating definite hepatocellular carcinoma. For LR-4 lesions or other lesions with an inconclusive pattern on imaging, biopsy is usually warranted.
Patients without cirrhosis or known chronic liver disease may require additional serologic testing for hepatitis viruses and tumor markers (eg, alpha-fetoprotein), as done in this patient. Although a serum alpha-fetoprotein level higher than 400 ng/mL in a high-risk patient is more than 95% specific for hepatocellular carcinoma, fewer than one-fifth of hepatocellular carcinoma cases are associated with such high alpha-fetoprotein levels.21 In our patient, although MRI alone was diagnostic of hepatocellular carcinoma, liver biopsy was performed owing to her unusual presentation.
CASE CONTINUED
Bone marrow biopsy was performed to evaluate the peripheral blasts and was normal. We attributed these findings to the patient’s underlying disease.
THE CHILD-PUGH SCORE AND ITS POTENTIAL PITFALLS
To determine candidacy for treatment of her hepatocellular carcinoma, a Child-Pugh score was calculated.
3. The Child-Pugh score is based on which combination of clinical criteria?
Total bilirubin, albumin, nutritional status, ascites, and encephalopathy
Total bilirubin, albumin, prothrombin time (PT) and international normalized ratio (INR), ascites, and encephalopathy
Total bilirubin, PT/INR, creatinine, sodium, and need for dialysis in the last week
Total bilirubin and PT/INR
Introduced in 1964, the Child-Turcotte-Pugh classification is a scoring system originally used to predict operative mortality and variceal bleeding risk in cirrhotic patients undergoing portocaval shunt surgery.22 It is based on total bilirubin, albumin, nutritional status, ascites, and encephalopathy. In 1973, the system was revised to include PT/INR instead of nutritional status.23
Since its inception, the Child-Pugh score has become an important tool for prognostication in patients with cirrhosis and determination of the necessity of liver transplantation. Additionally, it is used in patients with metastatic hepatocellular carcinoma to estimate severity of liver dysfunction and guide treatment options. Child-Pugh scores range from A (mild) to C (severe).
The Model for End-stage Liver Disease (MELD) score, based on total bilirubin, prothrombin time, creatinine, sodium, and need for dialysis, is another scoring system for assessing liver function and was created to predict survival in cirrhotic patients undergoing transjugular placement of intrahepatic portosystemic shunts.24 Like the Child-Pugh score, the MELD score is also used to estimate short-term risk of death, and helps with the prioritization of liver transplants.
Unrelated to the management of cirrhosis and hepatocellular carcinoma is the Maddrey discriminant function formula, which is based only on total bilirubin and PT and predicts benefit from steroid administration in patients with alcoholic hepatitis.
HEPATOCELLULAR CARCINOMA: STAGING, PROGNOSIS, AND TREATMENT
While the Child-Pugh score is a predictive model used most commonly in patients with cirrhosis, it is also used in patients with hepatocellular carcinoma to help determine candidacy for resection and systemic therapy in the metastatic setting.25 Although most established clinical trials of systemic therapy for metastatic hepatocellular carcinoma have been conducted in patients with Child-Pugh grade A cirrhosis, given concern for the competing risks of mortality and poor hepatic drug clearance due to liver dysfunction,26,27 there is a growing effort to include Child-Pugh grade B patients.28–30 It is important to note that the use of the Child-Pugh score for assessment of liver dysfunction is clinician-dependent, whereas other tools such as the MELD score and the albumin-bilirubin grade have also been used.15
The Child-Pugh score is also used in the Barcelona Clinic Liver Cancer algorithm, which is the most commonly used staging system and included in the consensus guidelines for the management of hepatocellular carcinoma.25,31 The Barcelona system categorizes patients into 1 of 5 stages, accounting for liver function (determined by the Child-Pugh score), tumor burden, and performance status.32 Accordingly, the Barcelona stages range from stage 0 (very early disease) to stage D (end-stage disease), with stage 0 patients having preserved liver function, excellent performance status, and single lesions measuring no more than 2 cm, while terminal stage D patients have metastatic disease with poor liver function and functional status. Hepatocellular carcinoma is an aggressive tumor and is often diagnosed late in its course, with most patients having stage C and D disease and median survival ranging from 2 to 20 months after diagnosis.33,34
For patients who have localized and resectable disease with preserved liver function, the mainstay of therapy is surgery with curative intent. Other curative options include liver transplant and liver-directed approaches (eg, thermal ablation). Importantly, patients may even be placed on liver transplant lists on the basis of diagnostic imaging alone, provided that certain technical, protocol, and standardized reporting requirements are met. In this manner, the risk of bleeding and tumor-tract seeding seen with biopsy is minimized.35
Unfortunately, many patients are ineligible for transplant due to the extent of disease or severity of their underlying liver dysfunction. In such cases, noncurative treatments to slow disease progression are offered, including transarterial chemoembolization, transarterial radioembolization, stereotactic body radiation therapy, and systemic therapy.15 Since the development of targeted therapy agents, supportive care is no longer the only option for Barcelona stage C patients. Therapies that have been approved as first-line options for nonresectable hepatocellular carcinoma include the multikinase inhibitors sorafenib and lenvatinib25–27 as well as atezolizumab plus bevacizumab.36 At progression, there are many other therapeutic options including but not limited to checkpoint inhibitors, regorafenib, or cabozantinib.37 As patients with Barcelona stage D tumors have an extremely poor prognosis, management is focused on symptom control with best supportive care.
CASE CONCLUSION
Although this patient had a normal bilirubin level and INR and no ascites or encephalopathy, she was characterized as being in Child-Pugh stage B due to her hypoalbuminemia. However, her albumin level of 2.5 g/dL was believed to be due to anorexia and poor oral intake due to her mandibular mass rather than liver dysfunction. Additionally, in this case the applicability of the Child-Pugh score was limited, as she did not have evidence of cirrhosis.
Because her functional status was otherwise good, systemic therapy with lenvatinib was started after completion of palliative radiation to her mandible for pain control. Unfortunately, 2 months into treatment, the patient developed painful vertebral fractures from her metastatic disease, and she entered hospice care.
4. If this patient’s chronic hepatitis B had been diagnosed earlier, how would she have been appropriately screened for hepatocellular carcinoma?
CT of the abdomen and pelvis with contrast every year
CT of the abdomen and pelvis with contrast every 6 months
Hepatic ultrasonography every year
Hepatic ultrasonography every 6 months
HEPATOCELLULAR CARCINOMA: SURVEILLANCE
The goal of surveillance is to improve overall survival through early tumor detection in groups at risk. The definition of high-risk populations varies by societal group, but the general consensus is to screen all patients who have any of the following25,38,39:
Child-Pugh A or B cirrhosis
Child-Pugh C cirrhosis awaiting transplant
Active hepatitis B but no cirrhosis
A family history of hepatitis C
African or Asian descent
Chronic hepatitis C with advanced-stage fibrosis in the absence of cirrhosis.
The American Association for the Study of Liver Diseases recommends surveillance with ultrasonography every 6 months, with or without alpha-fetoprotein levels (threshold of 20 ng/mL). Imaging with dynamic contrast-enhanced CT or MRI of the abdomen is typically indicated only if ultrasonographic visualization is limited, or for further characterization of lesions 1 cm or larger, as these are highly suspicious for hepatocellular carcinoma. Lesions smaller than 1 cm are likely benign but are closely monitored every 3 to 6 months at the physician’s discretion.25 These screening methods allow for earlier detection of hepatocellular carcinoma, leading to a lower risk of death and more treatment options.
In summary, this case illustrates an unusual presentation of hepatocellular carcinoma, the most common primary malignancy of the liver. While metastasis usually occurs in abdominal lymph nodes, bone, adrenal glands, or lung, only a few case reports have described spread to the mandible and maxilla.40–42 This case also demonstrates the importance of early recognition and detection of risk factors for hepatocellular carcinoma, including hepatitis B and C, particularly in diverse patient populations, as appropriate screening may lead to earlier diagnosis and better prognosis and outcome.
TAKE-HOME POINTS
Liver cancer is the fourth most common cause of cancer-related death worldwide, and hepatocellular carcinoma accounts for 80% of cases.
Mandibular metastases are rare and suggest aggressive, widespread underlying malignancy.
Hepatitis B is the main cause of hepatocellular carcinoma worldwide, whereas hepatitis C is the most common cause in Western countries. While cirrhosis is generally a prerequisite for the development of hepatocellular carcinoma in patients with hepatitis C, hepatitis B can progress to hepatocellular carcinoma without cirrhosis.
Patients at high risk for developing hepatocellular carcinoma, including patients with cirrhosis from any etiology and patients with hepatitis B with or without cirrhosis, should undergo surveillance for hepatocellular carcinoma with hepatic ultrasonography every 6 months.
The Child-Pugh score plays an important role in estimating the severity of liver dysfunction, which affects the prognosis and management of hepatocellular carcinoma. At the same time, it is important to recognize other confounding clinical variables that may affect the total score.
DISCLOSURES
Dr. Cho has disclosed consulting, teaching, and speaking for Bristol Myers Squibb and consulting for Eisai, Exelixis, and Genentech/Roche. The other authors disclose no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.