A 71-year-old man who had been previously well was brought to the emergency department by ambulance after experiencing several days of confusion and low back pain and a fall while showering. It was difficult to obtain a history owing to his altered mental status, but he said he had no chest pain or shortness of breath. His medical records were not available.
The patient’s temperature was 36.8°C (101.5°F), pulse rate 98 beats per minute, respiratory rate 16 breaths per minute, and blood pressure 100/60 mm Hg. He was somnolent but had no focal weakness or sensory deficits. Cardiac and pulmonary examinations were normal.
An electrocardiogram in the emergency department revealed ST-segment elevation in leads II, III, and aVF and ST-segment depression in V1, V2, V3, and aVL (Figure 1).
The patient’s electrocardiogram in the emergency department showed ST-segment elevation (arrows) in leads II, III, and aVF, and ST-segment depressions (triangles) in V1, V2,, V3,, and aVL.
FURTHER STUDIES
Laboratory test results were as follows:
White blood cell count 12.0 × 109/L (reference range 3.9–11.7) with a neutrophilic predominance
Hemoglobin 13.7 g/dL (13.3–17.7)
Creatinine 1.3 mg/dL (0.7–1.3)
Lactate 3.4 mmol/L (0.5–2.2)
High-sensitivity troponin I 48.3 ng/L (< 4).
Computed tomography (CT) of the brain did not show any acute abnormalities, and CT aortography excluded aortic dissection.
The patient was taken emergently for cardiac catheterization for ST-segment elevation myocardial infarction (STEMI). Coronary angiography revealed a left dominant circulation with the posterior descending artery and posterolateral branches arising from the left circumflex artery. A large filling defect was seen in the left dominant circumflex artery, and there were distal cutoffs in multiple obtuse marginal branches (Figure 2).
Coronary angiography revealed a left dominant circulation with the posterior descending artery and posterolateral branches arising from the left circumflex artery. A large filling defect was visualized in the left dominant circumflex artery as well as distal cutoffs in multiple obtuse marginal branches.
Balloon angioplasty followed by aspiration thrombectomy at the site of the filling defect improved coronary blood flow. The aspirated material had a yellow, organized, fibrinous appearance that was atypical for red thrombus (composed largely of red blood cells and clotting factors) or white thrombus (composed of platelets), both of which are seen during aspiration thrombectomy in acute coronary syndrome.1
Intravascular ultrasonography showed no evidence of atherosclerotic disease in the left circumflex or left posterior descending arteries. Intracoronary nitroglycerin did not increase the caliber of the coronary vessels or resolve the filling defects.
DIFFERENTIAL DIAGNOSIS
1. Which of the following is the most likely cause of the coronary artery obstruction in this patient?
Atherosclerotic plaque rupture with formation of intracoronary thrombus
Coronary embolization
Spontaneous coronary artery dissection
Coronary vasospasm
Atherosclerotic plaque rupture leading to formation of an intracoronary thrombus is the most common cause of STEMI, but in this patient, coronary angiography did not demonstrate significant coronary atherosclerosis, and intravascular ultrasonography did not reveal a culprit plaque that had ruptured, eroded, or fissured. Furthermore, thrombectomy of the occlusive coronary thrombus in the posterior descending artery returned fibrinous material that was not consistent with the appearance of a typical red or white thrombus of an atherosclerotic plaque rupture.
Spontaneous coronary artery dissection usually affects middle-aged women (90% of patients); it also accounts for 15% of myocardial infarctions during pregnancy or the peripartum periods.2,3 Furthermore, the patient had no angiographic or intravascular ultrasonographic evidence of artery dissection.
Coronary vasospasm can cause angina and infarction, often in association with ST-segment elevation. Angiography can reveal concomitant atherosclerosis or isolated vasospasm, neither of which was detected. The finding of occlusive filling defects on coronary angiography that improved with balloon angioplasty and aspiration excludes vasospasm as the primary cause of STEMI.
Embolism. The multiple distal cutoffs in the obtuse marginal branches are most consistent with an embolic event throughout the left dominant circumflex coronary artery.
CASE CONTINUED: RETURN FOR CATHETERIZATION
The patient was admitted to the coronary care unit. Over the next 24 hours, his temperature increased to 38.9 °C (102.0 °F), his white blood cell count increased to 29.7 × 109/L, and he developed hypoxic respiratory failure, acute kidney injury, and refractory shock despite antibiotics, vasopressors, and fluids.
Transthoracic echocardiography showed an ejection fraction of less than 20% and akinesis of the inferior, posterior, and lateral walls of the left ventricle. The patient’s clinical team thought that this degree of cardiac dysfunction was disproportionate to an inferoposterolateral infarct from a single lesion in the left circumflex artery and small distal cutoffs in obtuse marginal branches.
Given the unclear etiology of the patient’s shock and poor response to fluids, antibiotics, and vasopressors, he was taken back to the cardiac catheterization laboratory on hospital day 2 for right and left heart catheterization.
Right heart catheterization showed elevated right-sided and left-sided filling pressures, with the following values:
Pulmonary capillary wedge pressure 32 mm Hg (reference range < 12)
Cardiac index 1.7 (2.5–4.5)
Cardiac output 4.2 L (4–8)
Systemic vascular resistance 1,200 dynes/seconds/ cm−5 (800–1,200) on multiple vasopressors
Mean right atrial pressure 15 mm Hg (2–6).
Left heart catheterization showed normal flow through the previously occluded left dominant circumflex artery and its obtuse marginal branches. However, multiple new cutoffs were noted in the distal obtuse marginal arteries, consistent with repeat coronary embolization.
INTERPRETING THE HEMODYNAMIC MEASUREMENTS
2. The patient’s hemodynamic measurements point to which of the following mechanisms of shock?
Distributive
Cardiogenic
Mixed distributive and cardiogenic
Hypovolemic
Right heart catheterization is performed by advancing a balloon-tipped catheter through the right-sided chambers of the heart in the direction of blood flow and measuring filling pressures, oxygen saturation, and cardiac output. Cardiac output is calculated using the thermodilution or Fick method. Systemic vascular resistance is calculated based on the mean arterial pressure, central venous pressure, and cardiac output.
Hemodynamic measurements that can be used to determine the mechanism of shock (Table 1). The different types of shock include the following:
Types of shock
Distributive shock occurs when blood vessels are abnormally dilated, such as in sepsis or anaphylaxis. The cardiac output is usually normal or high, the systemic vascular resistance is low, and the pulmonary capillary wedge pressure is low.
Cardiogenic shock occurs when the heart fails to pump adequately, such as after myocardial infarction. The cardiac output is low, the systemic vascular resistance is high, and the pulmonary capillary wedge pressure is usually high.
Hypovolemic shock occurs when there is not enough volume in the intravascular space, such as after hemorrhage. The cardiac output is normal to low, systemic vascular resistance is normal to high, and pulmonary capillary wedge pressure is low.
The patient required multiple vasopressors to normalize his systemic vascular resistance, which indicated a vasodilatory state. The combination of low cardiac output and normal systemic vascular resistance on multiple vasopressors suggested mixed cardiogenic and distributive shock.
CASE CONTINUED: REFRACTORY SHOCK, NEW RESULTS
An intra-aortic balloon pump was placed to provide mechanical support to the left ventricle. However, the patient developed progressive hypotension and persistent lactic acidosis despite the balloon pump, inotropic support, vasopressors, mechanical ventilation, and continuous venovenous hemofiltration.
Repeat transthoracic echocardiography demonstrated progressive left ventricular dysfunction and a new, small pericardial effusion. No valvular dysfunction or vegetations were seen.
Repeat brain CT angiography demonstrated a new subarachnoid hemorrhage that was larger on the left side than on the right, and layered along the frontal sulci near the vertex without hydrocephalus or herniation.
Serial electrocardiograms demonstrated persistent ST-segment elevations in leads II, III, and aVF; new ST elevations in V4 and V5; and resolution of the ST depressions in aVL, V1, V2, and V3 (Figure 3).
On repeat electrocardiography 48 hours after presentation, the ST-segment elevations in leads II, III, and aVF were still present. The ST-segment depressions in aVL and V1-V3 had resolved, and new ST-segment elevations were present in V4 and V5 (arrows).
Blood cultures obtained on admission returned positive for Staphylococcus aureus.
INTERPRETING THE NEW ELECTROCARDIOGRAPHIC RESULTS
3. What is the most likely cause of this patient’s persistent ST-segment elevation at the 48-hour time point?
Recurrent ST-segment elevation myocardial infarction
Pericarditis
Ventricular aneurysm
Subarachnoid hemorrhage
Recurrent ST-segment elevation myocardial infarction. After STEMI, the ST-segment and reciprocal changes typically resolve within 48 hours. The persistence of ST-segment elevation after the reciprocal changes (ST depressions) had resolved was not consistent with recurrent STEMI in the same distribution.
Ventricular aneurysm or an akinetic ventricular wall can lead to persistent ST-segment elevation following STEMI, but neither finding was detected on echocardiography or angiographic ventriculography.
In subarachnoid hemorrhage, the most common electrocardiographic waveform changes are U waves and T-wave abnormalities. ST-segment elevation is not characteristic.4
Pericarditis. A key challenge at this juncture was distinguishing whether the patient was having a recurrent myocardial infarction or pericarditis. His initial electrocardiogram (Figure 1) was consistent with an inferoposterolateral myocardial infarction and could be explained by the distribution of emboli in the coronary anatomy. His left dominant coronary circulation supplied 3 major myocardial territories via the left circumflex artery and its branches: obtuse marginals supplied the lateral wall, the posterior descending artery supplied the inferior wall, and posterolateral branches supplied the posterior wall. ST-segment elevations in V6 were caused by emboli in the obtuse marginal branches supplying the lateral wall. Embolization into the posterolateral branches supplying the posterior wall caused depressions in V1, V2, and V3. Embolization in the posterior descending artery supplying the inferior wall caused elevations in II, III, and aVF and reciprocal depression in aVL.
In contrast, pericarditis is frequently associated with diffuse concave ST-segment elevation without reciprocal T-wave inversions or Q waves. (Occasionally, pericarditis manifests in focal leads).5 In this patient, the reciprocal depressions in aVL in the initial electrocardiogram had resolved, and new ST-segment elevations were present in V4 and V5 (Figure 3). The diffuse ST-segment elevations in a nonfocal coronary distribution, the absence of reciprocal ST depressions, and the presence of a new pericardial effusion were consistent with pericarditis.
CASE CONTINUED: A DEFINITIVE DIAGNOSIS
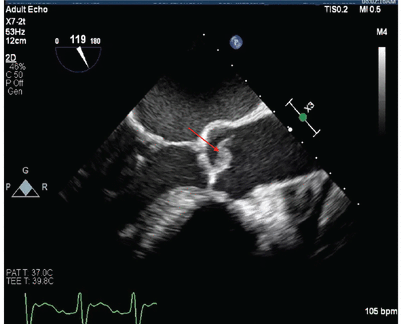
On hospital day 3, the patient underwent trans-esophageal echocardiography, which showed an 8-mm vegetation on the aortic valve without aortic regurgitation (Figure 4). The patient was diagnosed with S aureus endocarditis complicated by coronary and cerebral embolization; cerebral embolization was suspected to be the cause of the subarachnoid hemorrhage on CT. Magnetic resonance imaging of the spine excluded spinal infection.
Transesophageal echocardiography on hospital day 3 showed an 8-mm vegetation on the aortic valve (arrow).
The patient underwent aortic valve replacement. Intraoperatively, he was found to have purulent pericarditis, with pericardial fluid that grew S aureus. The aortic valve had a 1 cm × 2 cm vegetation on the non-coronary leaflet. A bioprosthetic aortic valve was implanted.
The patient could not be weaned from cardiopulmonary bypass and was placed on extracorporeal membrane oxygenation. On day 5 of hospitalization, he developed refractory sepsis and died.
SEPTIC CORONARY EMBOLIZATION
Infective endocarditis accounts for 1.58 million disability-adjusted life-years globally per year.6 Autopsy reports have shown that as many as 60% of patients who die with infective endocarditis have microemboli in the coronary circulation.7–9 However, only 3% to 11% of patients with infective endocarditis present with signs and symptoms of myocardial infarction attributed to macroemboli.8,10
The lower incidence of septic macroemboli causing coronary occlusion may be because of the brisk flow past the coronary ostia, the caliber differences between the aorta and the coronary arteries, the acute angle at which the coronary arteries branch from the aorta, and the favorable positioning of the coronary ostia behind the aortic valve cusps during systole, which may protect them from emboli.11 The risk of embolization is highest in patients with S aureus endocarditis and aortic valve endocarditis.12
Several case reports and small case series describe coronary embolism as a complication of infective endocarditis.8,10,13–15 A review found that a murmur was present in almost 90% of cases.8 In a case series, 13 of 14 patients had moderate to severe valvular regurgitation on echocardiography.14
Emboli most commonly enter the left coronary artery and travel to the left anterior descending artery, which generally has minimal angulation in its takeoff from the left main artery. In contrast, the circumflex artery typically branches at a 90-degree angle from the left main artery.8,11
Patients with STEMI from septic coronary emboli have a higher mortality rate than those with atherosclerotic myocardial infarction, both in the hospital (41% vs 3%–6%) and at 30 days (43% vs 2%–10%).8
Treatment
Evidence-based recommendations are available to guide antibiotic selection in infective endocarditis,16 but none exist on the management of septic coronary embolization.
Expert opinion supports early coronary angiography even when infective endocarditis is suspected, since coronary embolism is clinically indistinguishable from atherosclerotic plaque rupture at the time of presentation.17,18 Intravascular ultrasonography may be used to distinguish plaque erosion from embolus.
Aspiration thrombectomy with or without balloon angioplasty can be done to restore normal coronary blood flow.19,20 However, angioplasty with stenting may establish a nidus of infection in the vessel wall. Intracoronary thrombolytics, antiplatelet agents, and anticoagulants have also been used for small distal emboli, although these treatments carry higher risks of intracranial and systemic bleeding in patients with infective endocarditis.8,21
In this patient’s case, the initial presentation suggested typical STEMI due to atherosclerotic coronary artery disease. However, the angiographic, hemodynamic, and microbiologic data directed the clinicians toward a rarer cause of acute coronary syndrome—infective endocarditis. Infections of cardiac valves commonly cause morbidity through direct cardiac invasion and distant emboli. This patient’s case reminds us that emboli from heart valve vegetations sometimes take an unexpected turn.
TAKE-HOME POINTS
If angiography for STEMI does not reveal evidence of atherosclerotic plaque rupture, consider other causes of STEMI including coronary vasospasm, dissection, and embolization.
Fever, leukocytosis, and vessel cutoffs on angiography are early clues to septic coronary emboli; later test results, including blood cultures, vegetations on echocardiography, and mixed shock on hemodynamic measurements provide additional evidence for endocarditis.
Patients with STEMI from septic coronary emboli have higher in-hospital and 30-day mortality rates than patients with atherosclerotic myocardial infarction.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.