Oncologists who give immunotherapy today have had to rediscover internal medicine all over again. After immune checkpoint inhibitor therapy has been started, and even after it has been stopped, immune-related adverse events (irAEs) can manifest in any organ at unpredictable times and can replicate any naturally occurring autoimmune disease. But while irAEs can mimic their naturally occurring counterparts, their presentations, the ideal immunosuppressive regimens to control them, and their durations are unique. As such, irAEs are a new class of autoimmune disorders whose optimal diagnosis, management, and treatment remain incompletely discovered and ripe for innovation.
See related article, page 307
A SPECTRUM OF DISORDERS
The clinical spectrum of irAEs is broad. Any organ can be affected, and any naturally occurring autoimmune process can be mimicked by the massive inflammation generated by checkpoint inhibition. Common forms include immune-mediated diarrhea and colitis, inflammatory joint disease, hypothyroidism, and adrenal insufficiency. Unusual forms touch every organ system and range from hair repigmentation to aplastic anemia.1
Some irAEs are fatal. Perhaps the best described is the “triple M” syndrome, a highly morbid combination of myocarditis, myositis, and myasthenia gravis.2 Many phenomena that are likely immune-related have similarities to post-COVID-19 phenomenon, including brain fog, venous thromboembolism,3 and major adverse cardiovascular events.4
FIRST REPORTED IN 2006
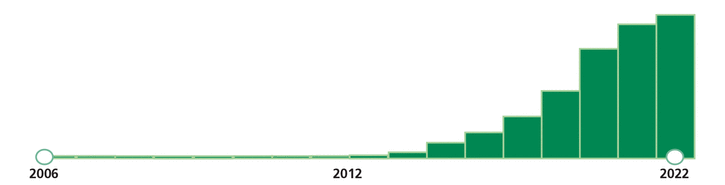
The first report of an irAE was logged in a pharmacovigilance database in 2006,5 as trials of checkpoint inhibitors got underway. In 2011, the modern era of cancer immunotherapy commenced with the approval of ipilimumab for metastatic melanoma. In 2012, 7 articles were published on irAEs and the first guide to practical management was published by the American Society of Clinical Oncology.6 Case reports and case series followed, as oncologists and medical subspecialists began to encounter these new disorders in their clinics and hospitals. These new experiences spurred a wave of publications (Figure 1), including a review of immune-mediated colitis in this Journal.7 In 2022 alone, 1,424 articles were published on the topic of immune-related adverse events. Since the first article on irAEs was published in 2007,8 more than 5,000 articles have been published on the clinical sequelae of checkpoint inhibition.
Timeline of articles on PubMed with the search term “immune-related adverse events.” More than 5,000 articles have been published since 2006 on a clinical phenomenon that did not exist before the introduction of checkpoint inhibitor therapy for cancer.
Dr. Kennedy and colleagues, in this issue of the Journal, present a welcome summation of endocrine dysfunction associated with checkpoint inhibitor therapy. Their article marches through all the flavors of checkpoint inhibitor-mediated endocrinopathy—the incidence, treatment, and optimal therapy, at least what has been published thus far. Endocrine irEAs are some of the most common long-term chronic sequelae of checkpoint inhibitor therapy.9
DIFFICULT QUESTIONS OF RISK VS BENEFIT
Confronting metastatic disease, an oncologist might dismiss the importance of possible endocrine irAEs, believing that permanent hypothyroidism is a relatively small price for inducing remission from stage IV disease. However, in the context of stage II or III cancer, particularly when surgical resection alone has high cure rates (eg, resected stage II melanomas, which have an over 70% cure rate with surgery alone5 and for which adjuvant anti-PD1 immunotherapy was approved in 2022), the prospect of permanent endocrinopathies makes the risk-vs-benefit calculation difficult for both patients and practitioners when deciding whether to begin a year of checkpoint inhibition.
INSURANCE HAS NOT CAUGHT UP
Despite the enormous medical interest in this new spectrum of clinical entities and despite medical recognition for well over a decade, irAEs do not exist in the medical insurance system: they do not yet have billable International Classification of Diseases codes. Obtaining prior authorization for biologic treatments that are recommended in major national and international guidelines is often challenging due to the nonexistence of appropriate billing codes that match the medical indication. Lack of proper reimbursement and prior authorization for biologic treatments is a significant barrier to optimizing treatment for irAEs and hampers the ability of the medical community to properly measure the medical, financial, and social impacts of this new class of disease.
STAY CURIOUS AND KEEP LEARNING
Every step in discovering the incidence of, inventing diagnostic algorithms for, and learning how to manage irEAs is a stride forward in the necessary and overdue education about the acute and chronic medical consequences of checkpoint inhibitor therapy for clinical practitioners in all disciplines, but most especially internal medicine, emergency medicine, and medical subspecialties. Lessons we have learned and continue to learn from these side effects, reinforced by the lessons from the COVID-19 era, have reminded us all in the medical specialties about the power and pleiotropy of the immune system. In these reincarnations of autoimmunity, we do as we always have done in medicine—constantly and consistently stay curious and renew our medical knowledge and practice.
DISCLOSURES
The author reports no relevant financial relationships which, in the context of her contribution, could be perceived as a potential conflict of interest.
- Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.