ABSTRACT
Immune checkpoint inhibitors are used more and more to treat several types of cancer, significantly extending cancer-free survival. However, concerns are growing about their toxic effects, which are many and varied. Endocrinopathies are some of the most frequently reported adverse effects, and thyroid dysfunction is the most common of these. Here, we review the incidence and severity of each immune checkpoint inhibitor-related endocrinopathy, possible factors related to toxicity risk, and principles of management.
The US Food and Drug Administration has so far approved 9 immune checkpoint inhibitors, which variously target programmed cell death protein 1, programmed cell death ligand 1, cytotoxic T-lymphocyte–associated protein 4, and lymphocyte activation gene 3.
Checkpoint inhibitor drugs have revolutionized cancer treatment, as they unleash the power of the immune system to destroy cancer cells.
Professional societies have issued guidelines for surveillance and treatment of immune checkpoint inhibitor-associated endocrinopathies.
With time and further research, strategies for predicting, preventing, and treating these toxicities should emerge.
The Discovery of the Molecular mechanisms by which cancer cells evade the immune system has brought about a revolution in cancer immunotherapy. In the past, immunotherapy had very limited success, but unmasking these mechanisms paved the way toward the invention of immune checkpoint inhibitors—monoclonal antibodies that block key regulators of the immune system. Cancer cells typically target these regulators, suppressing the immune response against them and thereby helping them evade the immune system.
See related editorial, page 318
Starting with ipilimumab in 2011, the US Food and Drug Administration (FDA) has so far approved 9 immune checkpoint inhibitors that target the following proteins:
Programmed cell death protein 1 (PD-1, less commonly known as CD279)
Programmed cell death ligand 1 (PD-L1, also known as CD274)
Cytotoxic T-lymphocyte–associated protein 4 (CTLA-4, also known as CD152)
Lymphocyte activation gene 3 (LAG-3).
These drugs have become mainstays in treating a variety of tumors, including those of the lung, esophagus, stomach, colon, liver, kidney, bladder, uterus, and skin.1 In fact, their efficacy has overtaken that of standard treatments, prolonging survival even in patients with tumors of advanced stage.
Nevertheless, concerns about the immune-related adverse effects of these drugs have been growing.2 The excessive activation of the immune system by these drugs causes dermatologic, endocrine, gastrointestinal, pulmonary, and other toxicities.2
In particular, endocrinopathies occur in roughly 10% of patients who receive immune checkpoint inhibitors.3 Hypopituitarism, type 1 diabetes mellitus, and thyroid and adrenocortical dysfunction are the most common disorders that checkpoint inhibitors cause, depending on the drug.4 The severity of these events has prompted researchers to look for adjuncts to minimize the toxicities while maintaining the efficacy of the drugs.3
Here, we review the mechanisms of action of the currently approved immune checkpoint inhibitors, the incidence of their associated endocrinopathies, the short-term and long-term outcomes of these adverse effects, and their management based on current guidelines.
MECHANISM OF ACTION OF IMMUNE CHECKPOINT INHIBITORS
The PD-1/PD-L1 pathway
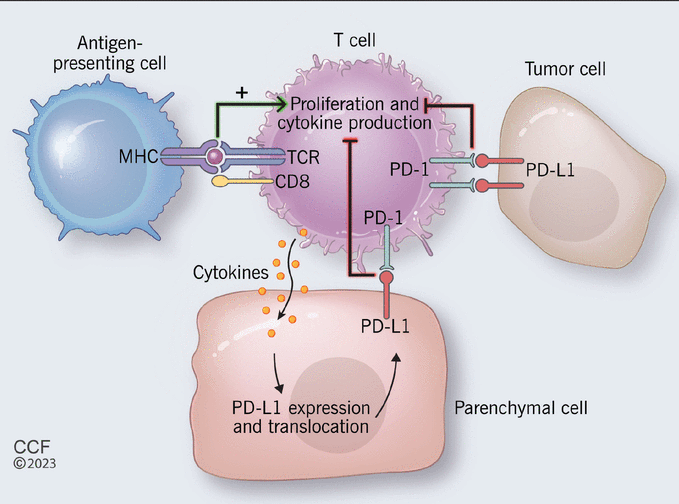
PD-1, a cell-surface protein, was discovered by Ishida and colleagues5 while studying apoptosis. It is most notably expressed by activated cytotoxic T cells after recognizing non-self antigens presented by major histocompatibility complexes of antigen-presenting cells.6 The interaction of the T-cell receptor and the major histocompatibility complex results in release of cytokines that trigger expression of PD-L1 by local parenchymal tissue.7 Parenchymal PD-L1 then binds T-cell PD-1 to transmit an inhibitory signal to the T cell and induce peripheral immune tolerance, so that healthy parenchymal tissue is protected from inflammatory destruction.6,7
Tumor cells manipulate this pathway by overexpressing PD-L1, so that T cells become exhausted and apoptosis is inhibited (Figure 1).6 Therefore, blocking either PD-1 or PD-L1 enhances cytotoxic T-cell activity against PD-L1–expressing cells, including those of both the tumor and the parenchyma.
Proposed mechanism of the programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 pathway (PD-L1). (MHC = major histocompatibility complex ; TCR = T-cell receptor.)
As of today, 4 PD-1 inhibitors (nivolumab, pembrolizumab, cemiplimab, and dostarlimab) and 3 PD-L1 inhibitors (atezolizumab, avelumab, and durvalumab) have been approved by the FDA.4
The CTLA-4 pathway
CTLA-4 is another T-cell surface protein that transmits inhibitory signals when bound by its ligands.8 It is homologous to T-cell receptor, which, in contrast, transmits stimulatory signals when bound. CTLA-4 binds the T-cell receptor ligands CD80/86 on antigen-presenting cells with greater affinity and avidity than T-cell receptor.9 Thus, it can outcompete T-cell receptor for its ligands and prevent its downstream stimulatory signal.
While this pathway is not manipulated by tumor cells, blocking CTLA-4 to ease T-cell receptor-CD80/86 binding enhances T-cell activation and cytotoxic activity against tumors.9 Ipilimumab remains the only FDA-approved CTLA-4 inhibitor to date.4
The LAG-3 pathway
The role of the LAG-3 pathway in tumorigenesis has been extensively studied since its discovery more than 30 years ago.10
LAG-3 is a transmembrane protein that binds major histocompatibility complex class II, suppressing proliferation and activation of T cells.10 This protein is also expressed on B cells and therefore has similar regulatory effects on B cells and natural killer cells.10 Naive T cells express low levels of LAG-3, but tumor antigens cause an increase in activity of LAG-3, leading to T-cell exhaustion.10
Inhibiting the LAG-3 pathway restores T-cell function, thereby leading to increased accumulation and effector function on tumor cells.10 Of note, combining LAG-3 inhibition with PD-1 blockade reduces tumor burden synergistically.11
In March 2022, the FDA approved the first human LAG-3 inhibitor (relatlimab), to be used in combination with nivolumab to treat unresectable or metastatic melanoma, based on data from a randomized phase 2 and 3 study.12
INCIDENCES OF ENDOCRINE IMMUNE-RELATED ADVERSE EVENTS
Pituitary dysfunction
Hypopituitarism is a rare endocrine disorder that can result from disease of the pituitary gland or the hypothalamus. Hypophysitis, ie, inflammation of the pituitary gland, usually leads to pituitary enlargement13,14 and has been reported to be a major cause of immune checkpoint inhibitor-mediated hypopituitarism, although some authors use the terms hypopituitarism and hypophysitis interchangeably.15 As the use of immune checkpoint inhibitors has increased in recent years, so has the incidence of hypophysitis.13,14
Immune checkpoint inhibitor-induced hypophysitis affects the anterior pituitary (which secretes follicle-stimulating hormone, luteinizing hormone, adrenocorticotropic hormone, thyroid-stimulating hormone, prolactin, endorphins, and growth hormone) more often than it affects the posterior pituitary (which secretes antidiuretic hormone and oxytocin),16,17 and most patients have multiple hormonal deficiencies. Barroso-Sousa et al3 reported in a meta-analysis that 36 (39%) of 92 patients on immune checkpoint inhibitor regimens who developed hypophysitis had symptoms of grade 3 or higher on the Common Terminology Criteria for Adverse Events (CTCAE) scale (Table 1).3,18
Common Terminology Criteria for Adverse Events (CTCAE)
Central hypothyroidism is the most frequent complication, followed by hypogonadism. This is distinctive with the CTLA-4 inhibitor ipilimumab, suggesting that CTLA-4 is expressed preferentially by the thyrotropin-secreting and gonadotropin-secreting cells.16,19
Central adrenal insufficiency is also common and is concerning, as it can lead to life-threatening adrenal crisis.
Hypoprolactinemia: Prolactin levels are usually low; hyperprolactinemia is uncommon.17
Growth hormone deficiency is rare, as the growth hormone axis is usually spared.14
Diabetes insipidus is a very rare feature of hypopituitarism.20
Risk factors for pituitary dysfunction
Male sex seems to play a role in incidence, with higher rates reported in men.16,17,21 Although this male predominance may be confounded by the sex discrepancies associated with melanoma (which also occurs more frequently in men, and which is treated with ipilimumab), the rates of hypophysitis still appear to be higher after taking this into account.16,17 This is in contrast to other etiologies of autoimmune hypophysitis, which are more common in women.14
Age is a contributing factor, with people over age 65 having a higher risk.16,17
Ipilimumab. Immune checkpoint inhibitor-induced hypophysitis-hypopituitarism is almost exclusively associated with the CTLA-4 inhibitor ipilimumab, and it appears to be the most common endocrinopathy associated with this drug,14 with incidences in the range of 10% to 15% reported.16,17 Cumulative dosage or cycle frequency do not appear to affect the incidence significantly.16
However, the incidence is significantly higher with nivolumab-ipilimumab combination therapy (about 8%) than with ipilimumab alone (about 4%).3
Hypophysitis-hypopituitarism occurs significantly less often with PD-1 inhibitors than with ipilimumab, and the presentations may drastically differ between the 2 drug classes, strongly suggesting independent pathways.22 For instance, gland enlargement and combined axis dysfunction are more common in those treated with ipilimumab, whereas secondary adrenal insufficiency with subtle gland enlargement is more common with PD-1 inhibitors.22
Human leukocyte antigen (HLA)-DR15. Due to the pathogenic nature of immune-related adverse events in general, predisposing HLA variants have been researched as a way to predict adverse outcomes. So far, studies have revealed an association between HLA-DR15 and the development of immune checkpoint inhibitor-induced secondary insufficiency.23
Course of hypopituitarism
The median time of onset of hypophysitis-hypopituitarism is 8 to 10 weeks after initiating treatment,16,17 although this can vary by as much as 4 months.24 Unlike other forms of autoimmune hypophysitis, it is usually not accompanied by visual disturbances.
Pituitary enlargement and hypophysitis usually resolve, but hypopituitarism can persist (with or without steroid treatment) and may be permanent depending on the hormonal axis involved.24 For example, the thyroid axis may recover in the long term, but recovery of corticotroph cell function is rare. Therefore, quality of life after immune checkpoint inhibitor therapy poses a major issue for patients with secondary adrenal insufficiency.
Thyroid dysfunction
Thyroid dysfunction is the most common endocrine immune-related adverse event associated with immune checkpoint inhibitor therapy. Dysfunction can be in the form of either thyrotoxicosis or hypothyroidism, but the latter is the more common presentation.13
Although some authors use the terms thyrotoxicosis and hyperthyroidism interchangeably, we would like to clarify the definitions. Hyperthyroidism is thyroid hormone overproduction caused by an intrinsic pathological excess in thyroid hormone synthesis and secretion by the thyroid gland, and examples are Graves disease, toxic adenoma, and toxic multinodular goiter. Thyrotoxicosis, however, encompasses all causes of thyroid hormone excess, including hyperthyroidism and pathologies that result in a temporary excess release of thyroid hormone, as we will describe later.
Thyrotoxicosis as the primary presentation is predominantly related to silent or destructive thyroiditis, and in most of these cases, hypothyroidism ensues shortly thereafter (median time 42 days).14,25 Therefore, many thyrotoxic adverse events may go undetected without close monitoring.14 For example, in a study by Lee et al25 of 45 patients who developed thyroid dysfunction after anti-PD-1 monotherapy or combination therapy, thyrotoxicosis was the initial presentation in 78% of patients, although 80% of those patients subsequently developed hypothyroidism.25 A study by Lu et al26 showed that only 9.3% of hypothyroidism cases reported to the FDA reporting system manifested with destructive thyroiditis (initially presenting as hyperthyroidism).
Graves disease is also an uncommon presentation of immune checkpoint inhibitor-induced thyrotoxicosis.13 However, due to inconsistency in reporting, the incidence of Graves disease as an adverse event may be underreported.13,27
Barroso-Sousa et al,3 in a meta-analysis of 38 randomized controlled trials, calculated the overall incidence of hypothyroidism to be 6.6% (95% confidence interaval 5.5%–7.8%) and the incidence of thyrotoxicosis to be 2.9% (95% confidence interval 2.4%–3.7%).3,14
Most recently, Lu et al,26 using data from the US food and Drug Administration Adverse Event Reporting System (FAERS) from 2011 until 2020, reported a much lower incidence of thyroid dysfunction—ie, 2.6%. Of these cases, 62% were hypothyroidism, 22.7% were hyperthyroidism, and 15.3% were reported as thyroiditis without thyroid function information.26 The authors accounted for this discrepancy as simply due to differences in study design and reporting, as clinical trials typically have closer follow-up and therefore more frequent reporting. Also, the FAERS reporting system may not capture less-severe adverse events. However, recent clinical data strongly suggest that the incidence of immune checkpoint inhibitor-induced thyroiditis and hypothyroidism is increasing,28,29 which is to be expected given the increased screening and reporting as well as increased use of immune checkpoint inhibitors.
Regarding severity, most of the thyroid-related adverse events are either asymptomatic (subclinical) or cause mild or moderate symptoms (CTCAE grade 1 or 2), with less than 1% leading to severe symptoms, hospitalization, or death (CTCAE grade 3 or higher).30
Risk factors for thyroid dysfunction
Women may have a higher risk of thyroid dysfunction than men.15,26,31,32 An explanation may be related to sex-hormone–mediated immune regulation, or possibly sex-specific autoimmunity.33
Elevated body mass index may also be associated with increased risk, earlier onset of symptoms, and overt hyperthyroidism.15,31
Advanced age may also increase the risk of severe thyroid dysfunction, leading to increased rates of hospitalization, morbidity, and death.26
Tyrosine kinase inhibitor use may also predispose to immunotherapy-related thyroid dysfunction,30,34 although tyrosine kinase inhibitors can also independently cause thyroid dysfunction.35
Biomarkers that may predict thyroid-related adverse events are elevated levels of thyroid-stimulating hormone, thyroid autoantibodies, thyroglobulin, and cytokines.15,31,36 In addition, Kurimoto et al36 demonstrated that higher serum levels of interleukin 1-beta, interleukin 2, and granulocyte-macrophage colony-stimulating factor at baseline and early decreases in interleukin 8, granulocyte colony-stimulating factor, and monocyte chemoattractant protein 1 were significantly associated with thyroid dysfunction (P < .5).36
Agent used. The incidence of thyroid dysfunction strongly depends on the type of agent and whether it is given as monotherapy or combined therapy. For instance, studies by Barroso-Sousa et al3 and Lu et al26 clearly demonstrated that the anti-PD-1 class poses the highest risk of thyroid dysfunction. On the other hand, the anti-CTLA-4 agent ipilimumab is associated with the lowest frequency of thyroid dysfunction.
Combination anti-CTLA-4 and anti-PD-1 therapy arguably has the highest risk of thyroid dysfunction. Of note, whereas previous studies consistently reported a higher incidence of thyroid dysfunction with combination anti-CTLA-4–anti-PD-1 therapy than with monotherapy of each class,3,4 Lu et al recently reported that the incidence of PD-1-related hypothyroidism exceeded that with combination therapy.26 However, this pattern of more adverse events with combination therapy is not unique to thyroid dysfunction, as discussed above with pituitary dysfunction. Also, higher CTCAE grades of thyroid dysfunction are more frequent with combination therapy.3,13
Dose and tumor type are not significantly associated with the incidence of immunotherapy-mediated thyroid dysfunction.3,13
Course of thyroid dysfunction
The time of onset of thyroid dysfunction varies greatly, within the first 15 weeks of therapy in most reported cases,37 but as early as 7 days or as late as 3 years in others.14 Also, the time to onset is shorter with combined immunotherapy than with monotherapy.25
Most cases of hypothyroidism remain permanent and require long-term levothyroxine replacement therapy.13,14
Pancreatic endocrine dysfunction, diabetes mellitus
The adverse effects of immune checkpoint inhibitor therapy on pancreatic endocrine function manifest in a similar manner to type 1 diabetes mellitus, with low or undetectable C-peptide and elevated autoantibody levels.14,38
Incidence rates have been reported to be between 0.2% and 0.9%, with 0.1% being CTCAE grade 3 or higher.3 However, there are recent reports of a more fulminant course with rapid-onset diabetic ketoacidosis39,40 associated with a disproportionately normal to mildly elevated hemoglobin A1c.38,41 Incidence rates are higher with PD-1 inhibitors (nivolumab, pembrolizumab), followed by PD-L1 inhibitors.38,41 Combination therapy may also increase risk, with a shorter onset of symptoms after initiation of therapy.38,41
Akturk et al,38 in a systematic review and meta-analysis of 71 cases, reported that the mean age of the patients was 61.7 (± 12 years), 55% of cases were in men, and the median time to onset was 49 days (range 5–448 days) after starting treatment. Half of the patients had autoantibodies at presentation, with a higher incidence of diabetic ketoacidosis and more rapid onset of diabetes mellitus than in patients without autoantibodies. An at-risk DR or DQ allele as present in 85% of patients tested, similar to the rate in childhood-onset diabetes.38
In a systematic review, de Filette et al41 reported comparable results, with similar incidences of diabetic ketoacidosis (71%) and islet autoantibodies (53%). However, fewer patients (65%) had susceptible HLA genotypes. These findings suggest a role of allele screening in patients who may be at risk of immune checkpoint inhibitor-induced diabetes.
As in childhood-onset type 1 diabetes, lifelong insulin therapy is needed, and unlike other immune checkpoint inhibitor endocrinopathies, pancreatic dysfunction does not respond to immunosuppressive therapy.42
Adrenal gland dysfunction
Immune checkpoint inhibitor-associated primary adrenal insufficiency is an infrequent manifestation of immune-related adverse events, accounting for less than 2%.
Barroso-Sousa et al,3 in their meta-analysis and systematic review, reported only 43 cases of any grade primary adrenal insufficiency among 5,831 patients (0.7%), of which 14 (0.3%) were grade 3 or higher. In another study, among 256 patients who received ipilimumab, 2 cases of primary adrenal insufficiency (0.8%) were observed.43
Grouthier et al44 reported that, of a total of 50,108 immune checkpoint inhibitor-associated adverse events (reported using the World Health Organization’s pharmacovigilance database of individual case safety reports over a decade), there were 451 cases of primary adrenal insufficiency, of which 45 were “definite” and 406 “possible.” A small majority (51.8%) of the cases were in men, with a median age of 66 years. Most patients had received immune checkpoint inhibitor monotherapy (nivolumab 44.3%, pembrolizumab 11.7%, and ipilimumab 23.6%), and 17.9% had received combination therapy. The median time to onset was 120 days (range 6–576).44
Immune checkpoint inhibitor-associated primary adrenal insufficiency is associated with significant rates of morbidity (> 90% of cases are severe) and mortality (7.3%). Mortality rates were similar in the subgroups receiving combination therapy vs monotherapy.44
Melanoma recurrence may also be a concern, due to persistently elevated adrenocorticotropic hormone and melanocyte-stimulating hormone levels, although no studies to date have fully investigated this theory.45
GUIDELINES FOR MANAGING ENDOCRINOPATHIES
The American Society of Clinical Oncology,49 the National Comprehensive Cancer Network,50 and the Society of Immunotherapy of Cancer51 have published practice guidelines on the recognition and management of immune checkpoint inhibitor-related endocrinopathies. The guidelines have similarities, but those of the American Society of Clinical Oncology are the most comprehensive, addressing acute management by CTCAE grade as well as when to consider endocrinology referral for thyroid dysfunction (Table 2), hypopituitarism (Table 3), adrenal insufficiency (Table 4), and diabetes (Table 5).49
Thyroid dysfunction due to immune checkpoint inhibitors: American Society of Clinical Oncology guideline
Hypopituitarism due to immune checkpoint inhibitors: American Society of Clinical Oncology guideline
Adrenal dysfunction due to immune checkpoint inhibitors: American Society of Clinical Oncology guideline
Diabetes due to immune checkpoint inhibitors: American Society of Clinical Oncology guideline
The American Association of Clinical Endocrinology52 has also published a clinical review on the evaluation and management of immune checkpoint inhibitor-mediated endocrinopathies, which shares a similar approach. However, it recommends a low threshold for endocrinology referral in the event of any laboratory derangement suggesting endocrine organ dysfunction. This includes thyroid dysfunction, as closer monitoring and further tests for adrenal insufficiency may be warranted.
Generally, unless patients have moderate or severe symptoms (CTCAE grade 3 or 4), immune checkpoint inhibitor therapy can continue throughout the endocrine adverse event. Permanently stopping these drugs is not routinely recommended,49 as most endocrinopathies are long-term and are treatable. This is unlike other immune-related adverse events (eg, pulmonary toxicities), for which more aggressive temporary or permanent discontinuation is recommended.49
Also, unlike in other organ-specific immune-related adverse events, steroids are not routinely recommended except in hypophysitis and primary adrenal insufficiency. High-dose steroids do not improve recovery rates in patients with hypophysitis53 and have been associated with worse outcomes.54 Therefore, high-dose steroids are reserved for patients with associated mass-effect symptoms. Similar outcomes have also been shown in patients with immune checkpoint inhibitor-induced diabetes,42 and steroids are generally avoided.
Dehydroepiandrosterone replacement is controversial. However, deficiency can be tested and treated in women with low libido or energy who are judged to be otherwise well-replaced for other hormonal deficiencies.
The adverse effects of immune checkpoint inhibitors do have an upside: they may predict that the drug is working on the cancer, and the patient can expect prolonged recurrence-free survival.55 In particular, endocrine immune-related adverse events (particularly thyroid dysfunction) may have the strongest associations with improved clinical outcomes.56 These correlations therefore further support the consideration of not discontinuing immune checkpoint inhibitor therapy once the adverse events are manageable.
AN EMERGING PICTURE
With the increased use of immune checkpoint inhibitors in cancer treatment, more precise reporting of their extensive endocrine immune-related adverse events is occurring. This will give a clearer picture of the true incidences and characteristics of each endocrinopathy and will help inform further updated guidelines for pretreatment investigations, monitoring, and management. Prolonged routine posttreatment monitoring should also be considered, as some adverse effects can occur many months after discontinuation.
Interspecialist planning and monitoring with endocrinologists and oncologists should also be considered, as this may allow earlier hormone-replacement therapy, as well as immunotherapy de-escalation for those with moderate to severe events.
Primary care physicians should be aware of the need to perform routine screening and surveillance investigations, when to consider endocrinology referral, and when to consider urgent or emergent care referral. Patients should be routinely and extensively counseled on the endocrine adverse effects, including the common signs and symptoms, when to seek urgent care, and the likelihood of indefinite hormonal replacement therapy should these events occur.
Finally, larger prospective studies are needed to answer questions pertaining to risk factors (age, sex, autoimmune markers), interventions to reduce risk of endocrinopathies, and the risk of recurrence after restarting therapy.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.