ABSTRACT
Venous thromboembolism (VTE) is a major health burden in patients with cancer, causing morbidity, emergency room visits, hospitalizations, and death. Treatment is challenging, as it is necessary to balance the risk of recurrent thrombosis and bleeding associated with anticoagulants. Treatment paradigms are shifting from low-molecular-weight heparin monotherapy. Multiple recent randomized controlled trials have demonstrated the safety and efficacy of direct oral anticoagulants in this setting. Current studies are evaluating factor XI inhibitors as potential treatments for cancer-associated VTE.
Patients with cancer are at a much higher risk of developing VTE than the general population.
Low-molecular-weight heparin or direct oral anticoagulants are preferred over vitamin K antagonists. Direct oral anticoagulants are generally preferred, but caution is needed in patients at risk of bleeding.
In the absence of bleeding concerns, anticoagulants should be continued for at least 6 months if the patient still has active cancer or metastatic disease or continues to receive systemic therapy.
Venous thromboembolism (VTE) events, including deep vein thrombosis, pulmonary embolism, and visceral vein thrombosis, are common in patients with cancer and can have significant consequences. In a study of 4,466 patients with cancer, thromboembolism (including VTE and arterial events) was reported to be the second major cause of death (tied with infection), after cancer itself.1 A recent large registry study showed higher rates of mortality, recurrent VTE, and bleeding in patients with active cancer when compared with patients with a history of cancer or no cancer.2 Sharman Moser et al3 compared patients with cancer with and without VTE and found those with VTE were more likely to be hospitalized (81.4% vs 35.2%), had longer hospital stays (20.1 days vs 13.1 days), and were more likely to visit the emergency room (41.5% vs 19.3%). Studies have shown a 39.5% increase in total healthcare costs in ambulatory patients with lung cancer and VTE,4 as well as increased healthcare utilization, a 3-fold increase in the rate of hospitalization, and an annual increase in per-patient cost of approximately $29,000 for recurrent VTE.5
The pathogenesis of the thrombophilic state in patients with cancer is distinct from that in populations without cancer and is multifactorial.6,7 Tumor cells can interact with host cells including endothelial cells, neutrophils, platelets, and monocytes. They promote the release of procoagulant factors and inflammatory cytokines that mediate endothelial dysfunction, including tumor necrosis factor alpha and interleukin-8.6 Certain factors also activate the coagulation cascade and remodel fibrin clot formation.7–9 Certain types of cancer can lead to leukocytosis and increased generation of neutrophil extracellular traps that capture and activate platelets, increase tissue factor activity, and secrete proteinases that promote metastasis. Another mechanism is cancer-associated thrombocytosis.6,7,10
PRESENTATION OF VTE IN CANCER
VTE develops in 5% to 20% of patients with cancer, and approximately 20% of all VTE cases occur in patients with cancer.11 Clinical and biologic factors that increase the risk of thromboembolism in patients with cancer include site of cancer, advanced stage (metastatic), use of central venous catheters, and treatment such as antiangiogenesis, chemotherapy, immunotherapy, surgery, hospitalization, and transfusion.7,8,11
VTE rates in patients with cancer are 4 to 7 times higher than in healthy individuals and are rising, possibly due to improved survival outcomes, use of thrombogenic cancer treatments (antiangiogenic agents, tyrosine kinase inhibitors, lenalidomide-based regimens, thalidomide), extensive use of central catheters, and increasing awareness.12–14 Studies have shown the highest risk of VTE is in patients with pancreatic and brain cancers, although risk is considered high in patients with gastric, esophageal, ovarian, and hematologic malignancies, particularly multiple myeloma and non-Hodgkin lymphoma.15,16 Because the prevalence of breast, prostate, and colorectal cancer is much higher, these cancers contribute to a significant proportion of VTE, despite having a lower relative risk.14
The risk of VTE recurrence is high even with administration of anticoagulation therapy, and various risk-assessment models are used to predict the risk in patients with cancer. Louzada et al17 studied 543 patients with cancer and VTE and formulated the Ottawa model to predict risk of VTE recurrence, which was later validated. Findings from the Computerized Registry of Patients with Venous Thromboembolism (RIETE)18 demonstrated the following risk factors for VTE recurrence: age less than 65, pulmonary embolism as initial VTE, and less than 3-month interval between cancer diagnosis and initial VTE.18
Deep vein thrombosis in patients with cancer mostly affects the veins in the lower limbs and usually presents as painful swelling and redness of the affected limb.7,19 Physical examination findings may include unilateral erythema, warmth, tenderness, difference in calf or thigh circumference, dilated superficial veins, and localized pain along the course of the involved vein. Rarely, patients can develop deep vein thrombosis in the internal jugular vein that can present as neck pain, swelling, erythema, headache, blurred vision, dizziness, and even altered sensorium. Other unusual sites of VTE include splanchnic, mesenteric, and portal veins that can present as abdominal pain, ascites, or gastrointestinal tract bleeding, but these are most commonly found incidentally on staging or restaging scans for malignancy. VTE in cerebral veins may present as focal neurologic deficits or seizures.20 The use of central venous catheters predisposes patients to upper-extremity deep vein thrombosis that presents with features similar to those of lower-limb deep vein thrombosis.21
Pulmonary embolism in cancer
Pulmonary embolism is another form of VTE presentation and can be a cause of sudden death.7,22–26 Common symptoms include shortness of breath, chest pain that is worse on inspiration (pleuritic type), cough, orthopnea, calf pain or swelling, and hemoptysis. On examination, pulmonary embolism can present with tachycardia, tachypnea, rales, decreased breath sounds, loud S2 heart sound, and jugular venous distention, as well as the S1Q3T3 pattern on electrocardiography (large S wave in lead 1, Q wave and inverted T wave in lead 3). This pattern indicates right ventricular strain and is rarely found in patients.
A recent study reported that patients with hematologic malignancies were less likely to develop pulmonary embolism (46% vs 55%) but had a higher risk of upper-extremity deep vein thrombosis (25% vs 18%) than patients with solid malignancies.22
Pulmonary embolism identified on contemporary imaging ordered for staging or restaging of primary cancer is termed incidental pulmonary embolism.7,23–26 VTE can also be the first manifesting feature of underlying malignancy.25 The rate of occult cancer may reach 10% at 12 months after the first unprovoked VTE event.25
In patients with cancer, VTE can be difficult to diagnose owing to overlapping symptoms, especially in patients receiving anticancer therapy,7,26 with a large number of symptoms misattributed to the underlying malignancy rather than to VTE.12,13
DIAGNOSIS
An elevated D-dimer is nonspecific, especially in patients with cancer, as it can be elevated without thrombosis.7,26 The high prevalence of VTE in patients with cancer decreases the negative predictive value and undermines clinical prediction rules in these patients.26 Pretest probability based on the Wells or Geneva score is used to guide evaluation for pulmonary embolism.7,27 Patients at low or intermediate risk can be evaluated with the highly sensitive D-dimer assay, age-adjusted cutoffs, and no further testing if negative. However, some experts consider imaging for patients with intermediate risk even if the D-dimer is negative. If the D-dimer is positive, computed tomography pulmonary angiography is warranted, although ventilation-perfusion scan is preferred to limit radiation exposure and for patients with contrast allergy or renal failure. If the patient is at high risk based on pretest probability (Wells or Geneva scores), computed tomography is warranted and D-dimer is not necessary prior to imaging.7,27
Although the Wells score classifies patients as likely or unlikely to develop deep vein thrombosis and recommends D-dimer testing or ultrasonography based on the score, compression ultrasonography is the mainstay for diagnosing deep vein thrombosis. Because the prevalence of VTE is high in patients with cancer and has worse outcomes, there is a low threshold for diagnostic workup or compression ultrasonography for deep vein thrombosis of the extremities.
Despite low or intermediate risk based on pretest probability, proceeding with imaging is appropriate if clinical suspicion for VTE is high, as is common in patients with malignancy.26,27
The rate of incidental deep vein thrombosis in the extremities varies from less than 1% to as high as 7% and may significantly underestimate the actual prevalence, as systematic assessment of distal veins may not always be performed.28 A recent meta-analysis showed the overall frequency of incidental pulmonary embolism to be 3.36% (95% CI 3.15%–3.57%), with variation depending on the site of the primary malignancy.29
TREATMENT
Appropriate treatment of VTE in patients with cancer is a challenge owing to the need to balance bleeding risks with the increased risk of recurrent VTE.30–37 The mainstay of therapy is anticoagulation.7 The type of cancer, thrombocytopenia due to cancer therapy, drug-drug interactions with systemic cancer therapeutics, bleeding risk, and nausea and vomiting associated with ongoing chemotherapy can further complicate management regarding the choice of anticoagulant drug, emphasizing the need for individualization.7
ANTICOAGULANT THERAPY OPTIONS
Vitamin K antagonists
Vitamin K antagonists inhibit the synthesis of vitamin K-dependent clotting factors (II, VII, IX, X). Tradition ally, vitamin K antagonists (eg, warfarin) have been the mainstay of treatment in VTE.14 Because of the need for regular laboratory monitoring, the narrow therapeutic range, dietary restrictions, and drug-drug interactions with commonly used chemotherapy agents such as 5-fluorouracil and less predictable pharmacology, the current practice has shifted toward the use of low-molecular-weight heparin (LMWH) and direct oral anticoagulants (DOACs).
LMWH
LMWH treatments have more predictable pharmacokinetic properties and better biologic availability, especially in patients with concerns for chemotherapy-induced emesis.37 LMWH monotherapy has been the standard treatment for cancer VTE for the past 15 years.26,30,31,34 Owing to efficacy shown in randomized studies, guidelines have recommended LMWH over vitamin K antagonists in patients with cancer.30,31,38
The first large study to address the benefit of LMWH in patients with cancer was CLOT (Randomised Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients With Cancer),30 which randomized 672 patients with active cancer and acute symptomatic VTE to receive dalteparin 200 IU/kg subcutaneously for 5 to 7 days, followed by a coumarin derivative with a target international normalized ratio of 2.5 or dalteparin (200 IU/kg once daily for the first month, then 150 IU/kg) alone for 6 months. Analysis showed lower rates of recurrent VTE over a 6-month follow-up period in the LMWH group, with 8% of patients developing recurrent VTE compared with 15.8% in the vitamin K antagonist group (hazard ratio [HR] 0.48, 95% CI 0.30–0.77, P = .002).30 No significant difference in major bleeding (P = .27) or any bleeding (P = .09) was reported between groups.30 A decade later, a larger, global randomized controlled trial, The Comparison of Acute Treatments in Cancer Hemostasis (CATCH),31 compared outcomes with tinzaparin and warfarin and showed no statistical difference in rates of recurrent VTE or major bleeding, but it did identify a significant reduction in clinically relevant nonmajor bleeding in patients randomized to tinzaparin (P = .004).31 However, current concerns with LMWH include the inconvenient subcutaneous route of administration and higher cost (at least in the United States) that may contribute to reduced patient adherence.12
DOACs
DOACs include oral direct thrombin inhibitors (dabigatran) and inhibitors of factor Xa (apixaban, edoxaban, and rivaroxaban). Apixaban, edoxaban, and rivaroxaban have been studied in the treatment of VTE in patients with cancer, but there have been no cancer-specific data published with dabigatran for this indication. Oral route, fixed dosage, and no requirement for routine monitoring or dietary restrictions as with vitamin K antagonists have increased the use of DOACs for long-term management.32–36 However, DOACs have significant drug-drug interactions, particularly with inducers and inhibitors of cytochrome P450 3A4 and P-glycoprotein.7,32 Immune-modulating agents (tacrolimus, dexamethasone, cyclosporine), tyrosine kinase inhibitors (nilotinib), topoisomerase inhibitors (etoposide), hormonal agents (bicalutamide), anthracyclines (idarubicin), and antimitotic agents (vinblastine, paclitaxel) have been known to cause interactions with DOACs.32 Caution is needed when DOACs are used for treatment in conditions such as hepatic or renal impairment, thrombocytopenia, active mucosal lesions, and unresected mucosal tumors, or when administered together with antiplatelet therapy. DOACs have also been noted to increase the risk of bleeding in gastrointestinal and genitourinary cancers.11
Several randomized controlled trials have shown noninferiority of DOACs vs LMWH.32–34 The Hokusai VTE Cancer trial proved noninferiority of the oral factor Xa inhibitor edoxaban (DOAC) over dalteparin (LMWH) in 1,050 patients with active cancer.32 The primary end point (composite end point of first recurrent VTE or major bleeding within 12 months) occurred in 12.8% of patients in the edoxaban group vs 13.5% in the dalteparin group (HR with edoxaban 0.97, P = .006 for noninferiority).33 The rates of recurrent VTE were not significantly different between groups (7.9% vs 11.3%, HR 0.71, 95% CI 0.48–1.06, P = .09). The edoxaban group had a higher rate of bleeding (6.9% vs 4.0%, HR 1.77, 95% CI 1.03–3.04, P = .04), particularly in patients with gastrointestinal cancers, both resected and unresected (12.5% vs 3.6%, HR 4.0, 95% CI 1.5–10.6, P = .005).14,33
Anticoagulation Therapy in Select Cancer Patients at Risk of Recurrence of Venous Thromboembolism (SELECT-D) was a randomized, open-label, multicenter trial involving 406 patients with cancer and symptomatic or incidental pulmonary embolism or symptomatic deep vein thrombosis of a proximal lower extremity that compared outcomes with rivaroxaban and dalteparin over a period of 6 months.34 The rate of recurrent VTE was reduced in the rivaroxaban group, with no significant difference between groups for rate of major bleeding. However, the rate of clinically relevant nonmajor bleeding events was higher in patients randomized to the rivaroxaban group (13% vs 4%, HR 3.76, 95% CI 1.63–8.69).34
The Caravaggio trial35 analyzed outcomes in 1,155 patients with cancer and symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism randomized to receive either oral apixaban or subcutaneous dalteparin for 6 months. The primary outcome of recurrent VTE was higher in the dalteparin group, and contrary to the SELECT-D study,34 the bleeding rate was not higher in the apixaban group.35
A meta-analysis including 4 randomized controlled studies comparing DOACs and LMWH showed a reduced rate of recurrent VTE (relative risk ratio [RR] 0.62, 95% CI 0.43–0.91, I2 30%) without a higher likelihood of major bleeding (RR 1.31, 95% CI 0.83–2.08, I2 23%).36
Consensus treatment approaches
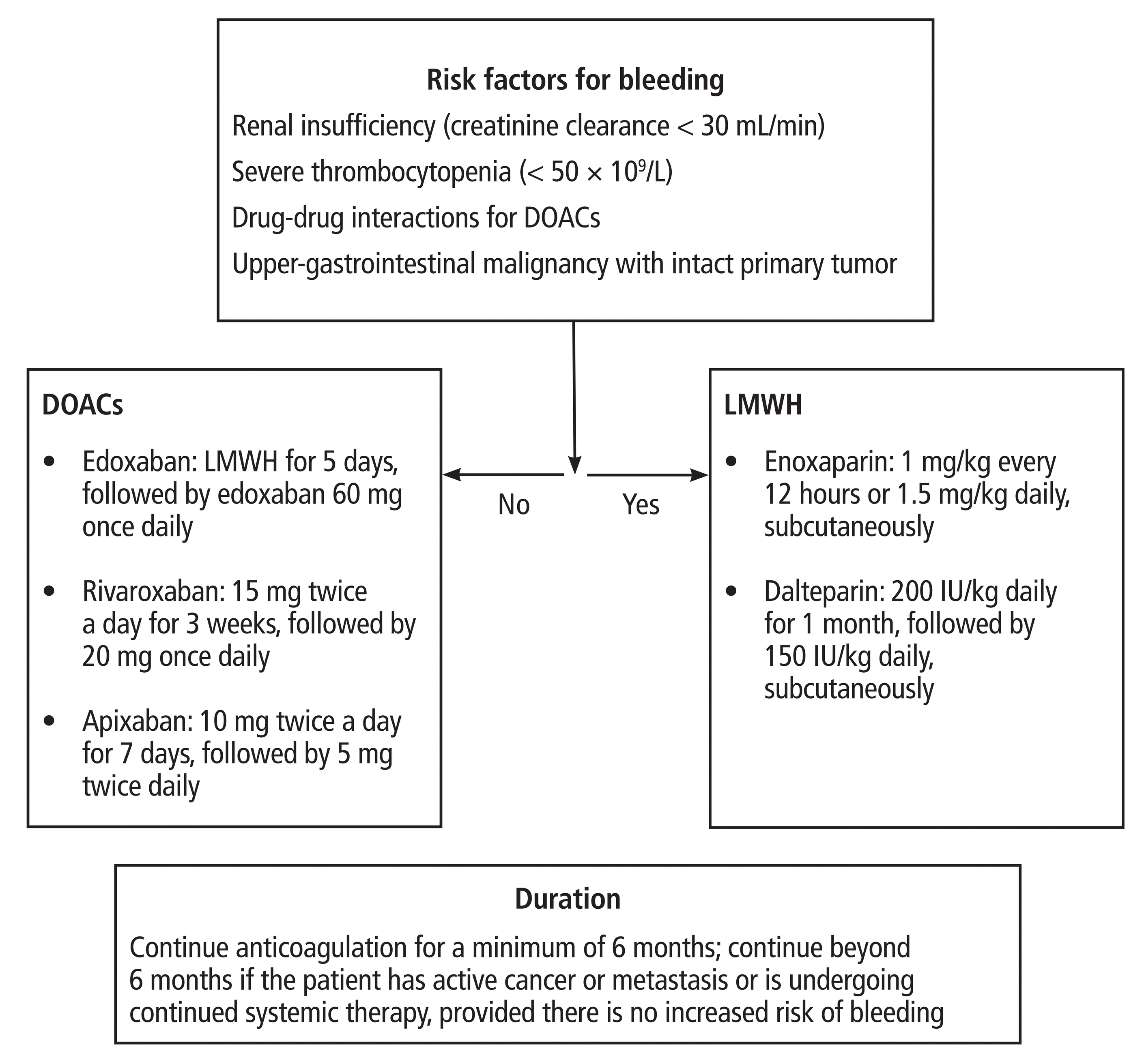
In general, guidelines from various societies regarding treatment of acute VTE in patients with active cancer show a substantial consensus.11,39–43 Both DOACs and LMWH are considered preferred treatment options. In the absence of risk factors such as renal failure, hepatic impairment, thrombocytopenia, drug-drug interactions, or upper-gastrointestinal malignancy with an intact primary tumor, DOACs are the preferred agents, whereas LMWH pharmaceuticals are preferred for those with these risk factors (Figure 1).11,16,32,36,39–43
There is a major knowledge gap regarding duration of treatment, as most clinical trials have focused only on the first 6 months of treatment. Current guidelines recommend that anticoagulants must be used for a minimum of 6 months and continued beyond at the same dose if the patient has active cancer or metastasis or is undergoing continued chemotherapy, provided there is no increased risk of bleeding. It is appropriate to use vitamin K antagonists in patients for whom access to DOACs or LMWH may be limited, such as in low-resource settings or for prohibitive copay costs. Treatment recommendations from various guidelines are summarized in Table 1.11,27,39–43
Guidelines for treatment of venous thromboembolism (VTE) in patients with cancer
SPECIAL CONSIDERATIONS IN TREATMENT
Incidental pulmonary embolism
Treatment is recommended for all incidental VTE (pulmonary embolism, deep vein thrombosis, multiple subsegmental pulmonary embolism).11 The American Society of Hematology recommends short-term anti-coagulation for 3 to 6 months for incidental pulmonary embolism in patients with cancer compared with observation alone.11 However, isolated subsegmental pulmonary embolism can be observed on a case-by-case basis without anticoagulant therapy in the absence of ultrasonography-detected lower-limb deep vein thrombosis.16
It is our practice to screen for lower-extremity deep vein thrombosis in the presence of isolated subsegmental pulmonary embolism before deciding about anticoagulation. The decision to start anticoagulation for incidental visceral vein thrombosis must be based on diagnostic certainty, chronicity, extent of thrombus, bleeding risk, and patient preference, but the certainty of evidence is very low.16
Recurrence during anticoagulation
Patient adherence, medication dosage, and the probability of heparin-induced thrombocytopenia must be correctly assessed if a patient develops recurrent VTE while on anticoagulation. These patients should be transitioned to a therapeutic dose of LMWH if on other anticoagulants, and their dose should be increased by 25% if LMWH was being used at a therapeutic dosage at the time of VTE.14,42 If the patient continues to experience recurrent thromboses, a further dose increase can be considered.42 In the rare case of anticoagulation failure or absolute contraindication to the use of anticoagulants (such as active bleeding), inferior vena cava filters can be considered.14,39,43 Retrievable filters are preferred and should be removed once contraindications to anticoagulation are safely addressed.11
Recurrence after stopping anticoagulation
As noted previously, anticoagulation must be resumed and continued indefinitely in the presence of risk factors such as active malignancy (ie, ongoing systemic therapy or metastatic disease), if there are no concerns for major bleeding risks.11,27,39–43 Recurrent VTE after cancer treatment should prompt evaluation for cancer recurrence or a new primary malignancy. DOACs or LMWH can be used and dose-adjusted based on bleeding risk for primary VTE.
Thrombocytopenia
Thrombocytopenia, defined as platelet count less than 100 × 109/L,16 can be the result of underlying malignancy or treatment with various chemotherapeutic agents. It is challenging to balance the risk of thrombosis and the risk of hemorrhage when managing patients with cancer and thrombocytopenia.16 LMWH is preferred in patients with thrombocytopenia, and studies are lacking regarding the safety of DOACs in such conditions. Samuelson Bannow et al44 reviewed studies involving 121 patients and found that prolonged thrombocytopenia increased recurrent VTE in patients with cancer. Further, they suggested that DOACs may not be appropriate for these patients, that unfractionated heparin is considered a reasonable alternative in certain settings, and that therapeutic or reduced-dose LMWH anticoagulation is an option.44 There was no significant difference in outcomes of recurrent VTE between the 2 treatment strategies, ie, therapeutic anticoagulation with platelet transfusion support or dose-modified anticoagulation if platelet counts were less than 50 × 109/L.44
With the risk of recurrent VTE highest within the first 30 days, full-dose anticoagulation for patients with platelet counts greater than 50 × 109/L is recommended.43 Patients with symptomatic segmental or proximal pulmonary embolism, proximal deep vein thrombosis, or history of recurrence should receive therapeutic-dose anticoagulation with platelet transfusion to maintain platelet counts above 40 to 50 × 109/L. Patients with incidental subsegmental pulmonary embolism or distal deep vein thrombosis can receive dose-modified anticoagulation (50% of the prophylactic dose of LMWH) for platelet counts between 25 and 50 × 109/L.43
After the initial 30-day period, a dose-modified strategy is suggested for platelet counts between 25 and 50 × 109/L.16,43,44 If the platelet count drops below 25 × 109/L, anticoagulation should be temporarily discontinued and then restarted once the count rises. Further, an inferior vena cava filter can be considered only for patients with contraindications to anticoagulants.
Renal insufficiency
Patients with creatinine clearance less than 30 mL/min have been excluded from many randomized controlled trials, leaving a lack of data on efficacy and safety of DOACs and therefore raising concern. A post hoc analysis of the CLOT trial showed a decreased rate of recurrent VTE with LMWH compared with vitamin K antagonists (HR 0.15, 95% CI 0.03–0.65, P = .01), but similar bleeding event rates for both treatments in patients with cancer and renal insufficiency (P = .47).45 Unfractionated heparin is an alternative to LMWH in patients with renal insufficiency.39
Distal deep vein thrombosis
VTE in veins distal to the popliteal vein (ie, the peroneal, anterior tibial, and posterior tibial veins) is considered distal deep vein thrombosis.46 Studies have shown that rates of bleeding and overall survival are similar in patients with isolated distal deep vein thrombosis and proximal deep vein thrombosis, and thus a treatment strategy similar to that for proximal deep vein thrombosis is recommended.46
CONCLUSION AND FUTURE DIRECTIONS
VTE leads to a significant health burden in patients with cancer. Anticoagulants such as DOACs and LMWH are the mainstay of treatment. Factor XI inhibitors are being developed in various settings for prevention and treatment of VTE. Abelacimab, a monoclonal antibody that inhibits factor XI activation and activity, is currently being studied in 2 randomized trials (ASTER trial NCT05171049, Magnolia trial NCT051710075) for treatment of acute VTE in patients with active cancer.47 Overall, drug development and treatment options have increased in the past decade in this setting, reducing the risk of recurrent VTE for patients with cancer. Given these options, treatment needs to be individualized for patients depending on the underlying malignancy burden, risk of bleeding, and patient preferences and values.
DISCLOSURES
Dr. Khorana has disclosed consulting for Anthos, BMS, Bayer, Genzyme/ Sanofi, and Pfizer, Inc; and consulting research for Anthos as principal or coinvestigator of funded research. The other author reports no relevant financial relationships which, in the context of her contributions, could be perceived as a potential conflict of interest.
Acknowledgments
Dr. Khorana acknowledges research support from the National Heart, Lung, and Blood Institute (U01HL143402, R01HL164516) and the Sondra and Stephen Hardis Chair in Oncology Research.
- Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.