ABSTRACT
Almost 40 million people worldwide are living with human immunodeficiency virus (HIV) infection. With treatment advances, HIV infection is now a manageable chronic disease for those with access to medical therapy. People living with HIV have a significantly higher risk and earlier onset of cardiovascular disease (CVD) owing to chronic inflammation and other biochemical factors, as well as overlapping social determinants of health and nonbiologic risk factors. Knowing that patients living with HIV develop coronary artery disease much earlier than the general population, careful attention must be given to assessment and management of their cardiovascular risk.
Because people living with HIV present with coronary artery disease about 10 years earlier than the general population, maintaining a higher index of suspicion for CVD, even in younger patients, is important.
Consider adjusting calculated CVD risk up 1.5 to 2 times and setting lower lipid targets and a lower threshold for starting statin therapy.
Selection of lipid-lowering agents must take into account any potential interactions with antiretroviral therapy medications; a multidisciplinary approach can be helpful.
Almost 40 million people worldwide, including more than 1 million people in the United States, are currently living with human immunodeficiency virus (HIV) infection.1,2 Over the past 50 years, scientific and policy advances have dramatically improved life expectancy for people living with HIV such that it is now a manageable chronic disease for those with access to medical therapy. As this population ages, clinicians must remain vigilant regarding comorbidities for which they are at heightened risk.
Specifically, people living with HIV have a significantly higher risk of developing cardiovascular disease (CVD). Globally, this risk is up to 2 times higher compared with individuals without HIV.3 While the concept that “undetectable equals untransmissible” is a tremendous breakthrough for people living with HIV, it is important for clinicians to remember that increased CVD risk persists even with control of the virus to undetectable levels. Herein, we review the epidemiology and proposed mechanisms of this phenomenon and discuss screening, management, and other considerations in treating people living with HIV.
CVD RISK PERSISTS DESPITE VIRAL SUPPRESSION
A model constructed using the data of more than 10,000 individuals being treated for HIV from a cohort of the AIDS Therapy Evaluation in the Netherlands suggested that, by 2030, 73% of people living with HIV will be 50 or older and 78% will have been diagnosed with CVD.4 Irrespective of viral suppression, this population has an increased relative risk of myocardial infarction, ranging from 20% to 100%, compared with people not living with HIV.5 Studies have shown that patients with HIV are also at increased risk for stroke, sudden cardiac death, heart failure, pulmonary hypertension, and myocardial fibrosis.3,6–9
The increased CVD risk in persons living with HIV persists even for patients taking viral suppressive therapies.10 A virtual cohort of the Veterans Aging Cohort Study—a multisite, longitudinal, prospective study—examined more than 80,000 patients living with HIV and noted that risk for acute myocardial infarction was higher in patients living with HIV in every age group.11 Importantly, this elevated risk remained when the analysis was restricted to patients with viral suppression. The Veterans Aging Cohort Study was relatively unique in that the patients were all male, the median age was 49 to 50, and 74% to 79% were Black or Latino.12 Similarly, a meta-analysis found that having HIV conferred a 61% increased relative risk of CVD in those not on antiretroviral therapy (ART).13 When limited to patients on ART, the relative risk of CVD was 2 times higher compared with patients without HIV.
PROPOSED MECHANISMS
The factors that contribute to the increased risk of CVD for patients with HIV infection are varied and not completely understood. Other recent studies reported that chronic inflammation and immune dysfunction persist even when the virus is well controlled.14
Lessons from studies of chronic inflammation in the general population
We know that chronic inflammation is linked to atherosclerosis. JUPITER (Justification for the Use of Statin in Prevention: An Intervention Trial Evaluating Rosuvastatin)15 showed that rosuvastatin reduced the incidence of cardiovascular events in patients with normal low-density lipoprotein cholesterol (LDL-C) but elevated high-sensitivity C-reactive protein (hs-CRP). Notably, chronic inflammatory biomarkers associated with atherogenesis are elevated in people living with HIV compared with those without HIV.14
IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial)16 analyzed the correlation of this dual target (LDL-C and hs-CRP) and the primary composite end point of cardiovascular death, major coronary event, and stroke for patients randomized to simvastatin monotherapy or a combination of simvastatin and ezetimibe.17 In a substudy that used the IMPROVE-IT data of 18,144 patients who had diabetes mellitus at randomization, simvastatin plus ezetimibe significantly increased the likelihood of achieving the predefined targets for LDL-C (< 70 mg/dL) and hs-CRP (< 2 mg/L). Further, a recent meta-analysis of randomized controlled trials showed that statins can be effective in reducing hs-CRP in patients with CVD, although further studies are warranted to clearly prove the beneficial effect of statins on hs-CRP.18
In a primary analytic cohort of the FOURIER study (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Patients With Elevated Risk), Bohula et al19 explored whether the association of inflammation and risk of cardiovascular events persisted even at very low levels of LDL-C. In patients with LDL-C less than 20 mg/dL 1 month after randomization, the 3-year primary event rate for patients with hs-CRP of less than 1, 1 to 3, and more than 3 mg/L was 9.0%, 10.8%, and 13.1%, respectively. This further supports the concept of an inflammatory risk for CVD. A secondary analysis from the CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcome Study) trial20 further supported this relationship between hs-CRP reduction and cardiovascular risk reduction.
While IMPROVE-IT, FOURIER, CANTOS, and JUPITER did not specifically focus on people living with HIV, the physiologic lessons learned regarding inflammation and treatment of CVD appear to be generalizable.
HIV-specific mechanisms
Hyperlipidemia. HIV infection itself is associated with a proatherogenic inflammatory state.21 The prevalence of hyperlipidemia in patients living with HIV ranges from 28% to 80%, compared with 10% to 11% among the general US population.22,23 Further, REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV),24,25 a large randomized, 12-country, multicenter trial, found that a daily statin (pitavastatin calcium) reduced major adverse cardiovascular events by 35% compared with placebo in people living with HIV aged 40 to 75 who had low to moderate CVD risk (with normal-range LDL).
HIV proteins. Even in patients with undetectable viral load on ART, low-level transcription of HIV genes encoding viral regulatory proteins continues.26 Such proteins, like transactivator of transcription protein and negative factor, have been shown to induce endothelial dysfunction as well as inflammation.27 Envelope glycoprotein 120, an HIV surface glycoprotein that helps the virus enter target cells, has been shown to stimulate production of the vasoconstrictor endothelin-1, which has been associated with cardiac morbidity and mortality.28
Cytomegalovirus infection. CD8 T-cell expansion and inflammation linked to cytomegalovirus coinfection is another mechanism that has been proposed for the enduring elevated cardiovascular risk in patients with HIV, even on ART.29
CD4 T-cell depletion and gut microbial translocation. Depletion of CD4 cells is associated with higher rates of myocardial infarction, ischemic stroke, heart failure, and peripheral artery disease.5 Replication of HIV in the gastrointestinal tract can severely reduce CD4 cells and thus lead to decreased function of the epithelial barrier.30 This allows microbial translocation and, in turn, a chronic inflammatory response. It is postulated that both the subsequent susceptibility to opportunistic infections from the depletion of CD4 T cells in the gut mucosa and microbial translocation lead to chronic states of inflammation.5
Nonbiochemical factors
It is important to acknowledge the myriad of nonbiochemical factors that may disparately affect people living with HIV and contribute to CVD risk. Owing to overlapping social determinants of health and other risk factors, the prevalence of cigarette smoking is 2 to 3 times higher in people living with HIV than in those not living with HIV.31
Further, transgender people are disproportionately impacted by HIV. The US Centers for Disease Control and Prevention reports an HIV prevalence of 9.2% in transgender people compared with less than 0.5% in adults overall.32 Studies have noted that metabolic changes asoociated with gender-affirming hormonal treatment may increase the risk of accelerated CVD.33,34 These factors, along with systemic healthcare factors that contribute to diminished access in these and other vulnerable groups, highlight the complexities of increased CVD risk.
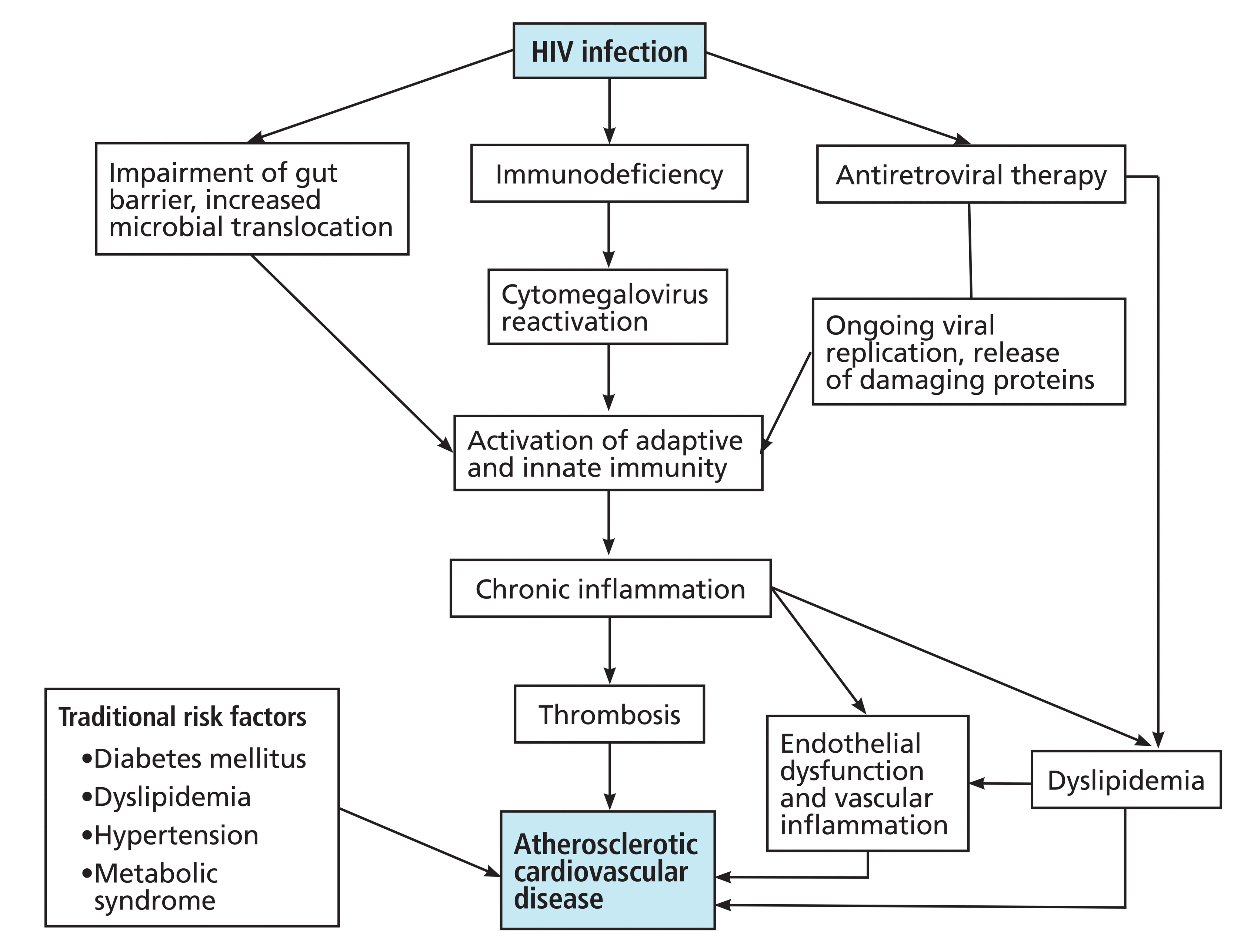
Even after adjusting for these and other various risk factors, however, studies have shown that people with HIV still have significantly higher CVD risk due to overlapping risk factors. Figure 1 illustrates the interplay of mechanisms of atherosclerosis and myocardial infarction in patients living with HIV.35 Figure 2 shows the proposed pathophysiologic mechanisms involved with HIV-associated atherosclerotic CVD.36
Mechanisms of atherosclerosis and myocardial infarction in people living with HIV.
aTraditional cardiovascular risk factors, such as smoking, and older therapies for treating people living with HIV also contribute to the development of atherosclerosis and myocardial infarction.
bMarkers of microbial translocation from the gut.
HIV = human immunodeficiency virus
Based on information from reference 35.
Pathophysiology of human immunodeficiency virus (HIV)–associated atherosclerotic cardiovascular disease.
Based on information from reference 36.
EARLIER ONSET OF CVD
An early study found that people living with HIV presented with coronary artery disease when they were about 10 years younger than patients without HIV.36,37 Further studies found these patients were more likely to have low thrombolysis in myocardial infarction risk scores and single-vessel disease but higher rates of restenosis after percutaneous coronary interven tion.36,38,39 The incidence of restenosis after drug-eluting stent placement in patients with HIV was 19% vs 10% in those not living with HIV—with CD8 count and C-reactive protein levels somewhat correlated.38,39
ART AND COMORBIDITIES
Early studies suggested ART as a possible contributing factor to the increased risk of CVD in people living with HIV. More recent studies, however, have noted that this is not the case with newer ART regimens. Herein, we examine the historical progression of evidence.
Dyslipidemia
Combination ART–associated dyslipidemia was first described in patients using protease inhibitors, regimens with nucleoside reverse transcriptase inhibitors, and nonnucleoside reverse transcriptase inhibitors, with duration of exposure being associated with increased CVD.40 Part of this has been attributed to some component of the initial return of appetite and weight gain in these patients once they started ART. The historic DAD (Data Collection on Adverse Events of Anti-HIV Drugs) trial,41 a comprehensive examination of CVD adverse events associated with ART, found an association between ART and dyslipidemia.
Older ART drugs, including abacavir, ritonavir, and lopinavir, have more cardiotoxic effects, including left ventricular dysfunction or altered lipid or glucose metabolism.42 Specifically, older protease inhibitors like ritonavir are known to induce hypertriglyceridemia and other adverse effects such as dyslipidemia, hyperglycemia, and overt diabetes mellitus.42 In addition, protease inhibitors boosted by ritonavir, as well as some first-generation nucleoside reverse transcriptase inhibitors and non-nucleoside reverse transcriptase inhibitors, have been shown to affect lipid parameters, such as increased total cholesterol, LDL-C, and triglycerides.43,44 One study found that exposure to the protease inhibitors lopinavir-ritonavir and indinavir or the nucleoside reverse transcriptase inhibitors abacavir or didanosine was associated with increased risk of myocardial infarction.42 This risk in patients taking abacavir appears, in some studies, to be linked to increased platelet reactivity and endothelial dysfunction.36
Further, different classes of ART can have differing effects on the development of adipose tissue. For example, some integrase inhibitors have been associated with weight gain, and some first-generation nucleoside reverse transcriptase inhibitors and protease inhibitors have been shown to correlate with the development of lipodystrophy.43,44
Newer ART medications, such as the C-C chemokine receptor 5 antagonist maraviroc and the integrase inhibitor raltegravir, are more favorable with respect to effect on lipid levels.22 One recent study showed that switching from a protease inhibitor to the newer integrase inhibitor bictegravir was associated with improvement in lipid markers.45 In particular, patients with the worst baseline lipid profiles had significant improvements, and those who switched from protease inhibitors to bictegravir also saw improvements in triglycerides. Dolutegravir, another integrase inhibitor, has a more neutral effect on lipids compared with efavirenze or ritonavir-boosted darunavir.46
Overall, initiation of ART may negatively impact lipid levels (including LDL, triglycerides, total cholesterol, and high-density lipoprotein), and this change is likely multifactorial. Lipid monitoring in patients living with HIV is imperative for CVD risk reduction.
Insulin resistance and metabolic impact
First-generation ART medications were associated with insulin resistance and metabolic syndrome, leading to a higher incidence of diabetes and elevated hemoglobin A1c levels in people living with HIV.43 Historically, protease inhibitors have been associated with insulin resistance, but these agents may be less commonly used due to other toxicities, such as the possibility of hepatotoxicity, Stevens-Johnson syndrome, or elevated cholesterol, as noted above. First-generation thymidine nucleoside reverse transcriptase inhibitors also impacted fat distribution and caused weight gain, effects that may be responsible for a lingering occurrence of insulin resistance in aging patients who used these medications.43
Evidence suggests that certain HIV preexposure prophylaxis regimens may be associated with metabolic changes (although the evidence is from trials that were not specific to people living with HIV).47,48 Specifically, initiation of tenofovir alafenamide fumarate for preexposure prophylaxis was associated with increased risk of hypertension and statin initiation, especially in patients 40 and older.47 Another study examined tenofovir disoproxil fumarate and emtricitabine and found a modest reduction in cholesterol.48 The participants in the first study were found to have weight gain, and the participants in the second study were found to have weight loss, which may be the independent driver regarding metabolic impact. These results may further inform future studies on the effects of ART drugs on metabolic function.
MANAGEMENT
Models underestimate CVD risk
The 2019 American College of Cardiology and American Heart Association guidelines49 on primary prevention of CVD recognized HIV as a risk factor for CVD based on the presence of chronic inflammation. In doing so, these guidelines acknowledged that standard models for predicting CVD risk systematically underestimate CVD risk for people living with HIV.10 A recent meta-analysis examined 9 major CVD risk-prediction models and found a general tendency for these models to underestimate risk in these patients.50
As such, the guidelines note that individuals living with HIV may benefit from a lower threshold for statin initiation or intensification, particularly those with intermediate risk (≥ 7.5% to < 20% 10-year atherosclerotic CVD risk).49 Further, consideration of the underlying HIV diagnosis may help to sway treatment decisions for patients with borderline (5% to < 7.5% 10-year atherosclerotic CVD risk) or intermediate risk.51
The American Heart Association scientific statement52 for prevention and treatment of CVD for people living with HIV suggests that clinicians may consider adjusting calculated CVD risk assessments up 1.5 to 2 times for patients with HIV, particularly for those with certain HIV-associated risk-enhancing factors like prolonged viremia, delayed ART initiation, and low CD4 count. This was more specifically discussed in the European Society for Cardiology guidelines,53 which also suggest an LDL-C goal of less than 70 mg/dL in people living with HIV.53
REPRIEVE, discussed earlier, examined strategies for CVD risk prevention in people living with HIV. The study’s results led to the concept that patients living with HIV should begin statin therapy to reduce CVD risk and that this should be individualized and communicated with the patient as part of shared decision-making.24 However, many patients with HIV who already meet criteria for statin use do not receive a prescription for them (28%) or are prescribed statin therapy below the indicated intensity (12%).54
Considerations when selecting lipid-lowering agents
Selection of lipid-lowering agents in patients on ART requires attention to potential drug-drug interactions. Ultimately, considerations should be patient- and case-specific and part of a multidisciplinary discussion with infectious diseases and pharmacy colleagues in the broader context of patient care.
Overall, we know that statin therapy can be administered safely in patients on ART.55 Some illustrative examples can be useful. Lovastatin and simvastatin are contraindicated in patients being treated with protease inhibitors because of the increased risk of rhabdomyolysis.56 One study suggested a higher risk for atorvastatin in patients taking both protease inhibitors and ritonavir,57 and the Infectious Diseases Society of America recommends starting with a lower dose of atorvastatin in these patients.58 Of note, proprotein convertase subtilisin/kexin 9 inhibitors have recently shown promise in reducing atherogenic lipid levels in patients living with HIV and may be of increased utility in the future as investigations continue.59
A NOTE ON HEART FAILURE IN PATIENTS WITH HIV
There is currently no difference in guidelines regarding treatment of heart failure in people living with HIV and those not living with HIV. Recent studies have, however, indicated that the increased risk of heart failure for patients living with HIV is not primarily mediated through atherosclerotic disease pathways.60 With respect to HIV-associated cardiomyopathy, the prevalence of systolic dysfunction has decreased with the spread of ART, but the number of patients with HIV with abnormal diastolic function has increased.61 One meta-analysis reported systolic and diastolic dysfunction incidence at 8.3% and 43.4%, respectively, in people living with HIV.62 Direct HIV-induced myocardial damage may have been a predominant driver of systolic dysfunction before the widespread use of ART, hence, a relative decline.61 Theories to explain the increase in diastolic dysfunction have included higher rates of inflammation, hypertension, or direct impact on myocardium.63
Mechanisms that have been proposed to explain the pathophysiology of HIV-associated cardiomyopathy outside of those behind atherosclerotic risk and acute coronary syndrome are, again, multifactorial.61 Direct HIV-induced myocardial damage, alluded to above, is one such mechanism. It is theorized that inflammation in the myocardium may contribute to increased left ventricular mass, which is consistent with studies examining similar findings in patients with other types of inflammation, such as systemic lupus erythematosus and rheumatoid arthritis.63 Moreover, chronic inflammation and immune dysfunction may lead to collagen deposition and fibrosis in the myocardium itself.64 While cardiomyocytes lack HIV-1 receptor proteins (glycoprotein 120 and 24), cardiac interstitial cells may serve as viral reservoirs and mediate inflammation.61,65 Other mechanisms include negative inotropic effects exerted by proinflammatory cytokines that contribute to reduced systolic function, autoimmune effects, and side effects from some ART medications.61
An approach to heart failure risk stratification for patients living with HIV could be beneficial going forward.66 Chowdhury et al67 are currently studying whether people living with HIV receive standard of care for heart failure compared with people not living with HIV, which could reveal areas for potential focus in the future.
AREAS FOR FUTURE WORK
The link between HIV infection and CVD risk is clear. What remains for discovery are the more granular factors that may increase risk in a subset of people living with HIV and how best to reduce this risk.6,52 One study examined various biomarkers in people living with HIV in an attempt to create different cluster phenotypes.68 Those in the cardiac phenotype (for example, with elevated interleukin-1 receptor–like protein) were more likely to experience pulmonary hypertension, and those in the inflammatory phenotype (for example, with elevated C-reactive protein and interleukin-6) were more likely to experience diastolic dysfunction. Such studies that examine biomarkers with greater granularity may guide future therapies and screening.
To this end, further studies examining factors associated with HIV infection—including hepatitis C virus coinfection, CD4 count, years of sustained and elevated viral load, history of opportunistic coinfections, timing of ART commencement, and others—are warranted and may yield a more standardized approach to risk analysis and management.
Finally, it is crucial that patients living with HIV be included in trials that study cardiac risk factors to broaden the applicability of evidence to this population. This will greatly aid the collective understanding of how to reduce CVD risk and prevent adverse events in patients living with HIV.
TAKE-HOME POINTS
Screening and treatment of CVD should be tailored to patients living with HIV due to their heightened risk profiles.
Because we know that patients living with HIV develop coronary artery disease much earlier, assessment of CVD risk should be considered along with potentially lower lipid targets and a lower threshold for treatment. Selection of lipid-lowering agents must take into account any potential interactions with ART medications, and a multidisciplinary approach is helpful.
Careful attention to other cardiovascular pathology is imperative for patients living with HIV, including diastolic and systolic heart failure.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2025 The Cleveland Clinic Foundation. All Rights Reserved.