ABSTRACT
Chronic venous outflow obstruction is a significant cause of chronic venous disease and therefore chronic morbidity. When conservative measures fail, intervention through deep venous reconstructive techniques should be considered. Referral should be considered in all patients with features of chronic venous disease that are life-affecting. Imaging relies primarily on duplex ultrasonography, supplemented by computed tomographic and magnetic resonance venography, and intraoperatively by intravascular ultrasonography. Intervention is primary endovenous, using angioplasty and stenting. Open surgical procedures are used in very select patients.
Chronic venous disease is common and costly in terms of physical discomfort and quality of life.
Chronic venous outflow obstruction is an important cause of chronic venous disease.
Although invasive and costly, intravascular ultrasonography is the gold standard for detection.
Early treatment including anticoagulation and other preventive measures reduces the likelihood of recurrent deep vein thrombosis.
Referral to a vascular specialist center with experience of deep venous reconstruction is recommended.
Recent advances in imaging and stent technology are changing the management of chronic venous outflow obstruction (CVOO), an important cause of chronic venous disease (CVD). Evidence increasingly supports endovascular intervention as a potentially effective and safe treatment option.
This article reviews the key factors to consider in management of CVOO and advises on how best to get patients the care they need.
CHALLENGES: QUALITY OF LIFE, TREATMENT OPTIONS
CVOO negatively affects quality of life and mental health. The presentation of CVOO can be similar to that of superficial venous incompetence, but proximal edema tends to be more significant in CVOO. Common manifestations include leg-swelling and pain, limited mobility, chronic ulceration, and venous claudication. Neglen1 and Raju2 estimated that such lesions occurred in up to 55% of patients with significant CVD, especially in those with postthrombotic syndrome (PTS). Recent reports suggest that CVOO may also contribute to chronic pelvic pain, including pelvic congestion syndrome,3 although this observation remains controversial and requires further study.
Consequently, patients are subjected to long-term pain and discomfort, the need for chronic leg ulcer management, and reduced physical activity.4 Healthcare systems therefore allocate significant resources for the treatment of CVOO and related CVD.5
Although endovenous and open surgical interventions are effective and safe treatments for superficial venous incompetence, mainstay management for CVOO until recently has been limited to compression therapy and supportive measures such as lifestyle changes. These nonsurgical measures are often unsatisfactory to patients as well as clinicians. Open deep venous reconstructive surgery also has limitations: it is invasive, evidence is insufficient to support the benefits, and its use is limited to a very select group of patients and surgeons.6 Endovascular intervention is a promising option.
Terminology
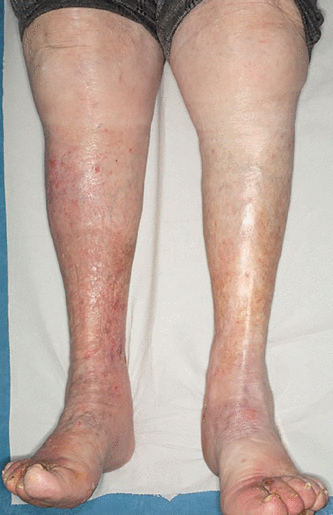
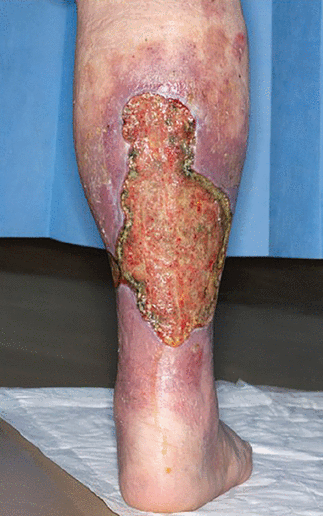
The definition of CVD is wide-ranging and patient-specific, often characterized by manifestations of chronic venous hypertension. Symptoms and signs include varicose veins, telangiectasias, pain and discomfort, cramps, restless legs, itching, heaviness, and edema. Skin changes can include venous eczema, lipodermatosclerosis, and ulceration (Figures 1 and 2), and explain why patients may consult or be referred to dermatologists instead of vascular specialists. CVOO often refers to long-standing stenotic and occlusive disease of the central veins, ie, iliofemoral veins or inferior vena cava (IVC) for the lower limbs, or both.
Venous eczema associated with chronic venous insufficiency of the lower limbs. The condition is worse on the right leg.
Severe venous ulceration associated with chronic venous insufficiency. Venous ulceration typically occurs in the ankle (gaiter) with surrounding skin changes such as venous eczema (purplish discoloration around the ulcer) and lipodermatosclerosis as well as edema. No clinical feature of the ulcer indicates that chronic venous outflow obstruction (CVOO) is the cause, but the severity of the disease is often worse with CVOO than with superficial venous incompetence, although not exclusive.
WHAT CAUSES CVOO?
CVOO can be thrombotic or nonthrombotic in origin.
Thrombotic CVOO is a long-term complication of deep vein thrombosis (DVT) involving the central veins, causing chronic occlusion or incomplete recanalization (stenosis), or both. There are varying degrees of collateral vein formation. The DVT can be associated with an underlying extrinsic compression, which can be malignant or benign.
Nonthrombotic obstruction also can occur, either benign or malignant. Benign lesions include nonthrombotic iliac vein lesions (NIVLs), uterine fibroids, and retroperitoneal fibrosis.
Recruitment of collateral veins to bypass an obstruction is often inadequate, partly due to their much smaller cross-sectional areas compared with the central veins. According to Poiseuille’s law, volumetric flow rate is related to the fourth power of the vessel radius. Therefore, CVOO causes reduced venous return from the lower limbs, which leads to repeated and long-standing venous stasis and pooling. As a result, chronic venous hypertension develops in the affected lower limb. This is thought to trigger inflammatory processes that affect the microcirculation, ultimately manifesting as CVD.
At the microvascular level, chronically elevated venous pressure leads to capillary fluid leak, basal membrane degeneration, inflammatory infiltrates, and a negative cycle of tissue degeneration and scarring. Poorly healing ulcers develop and can become chronically infected, leading to significant morbidity.
Postthrombotic syndrome
PTS is chronic venous disease that can occur in up to 50% of patients in the 2 years after DVT.7 It is a consequence of venous obstruction or valvular damage. Either or both can result from chronic inflammatory processes and inadequate venous recanalization following a DVT.
Several diagnostic and severity scales such as the Villalta-Prandoni scale (Table 1)8 are available to help diagnose and evaluate the severity of PTS. Venous ulcers can develop in up to 10% of patients in the 2 years following DVT.9 The severity of disease often correlates with the proximity of the DVT. For example, disease is worse in iliocaval and iliofemoral DVT than in femoropopliteal and calf DVT. Adequacy of immediate management is also a factor.5
Evaluating the severity of postthrombotic syndrome (PTS): The Villalta-Prandoni scale
Nonthrombotic iliac vein lesions
Nonthrombotic iliac vein lesion (NIVL) refers to extrinsic compression of the iliac vein. Up to 66% of the general population may have an asymptomatic NIVL,10 so a careful workup is needed to identify NIVL as the cause of disease. May-Thurner syndrome (also known as Cockett syndrome or iliac vein compression syndrome) is compression of the left common iliac vein at the site where it is crossed by the right common iliac artery.11 In some patients, the close, persistent pulsing of the right common iliac artery causes chronic extrinsic compression of the left common iliac vein with intimal scarring and fibrosis. Similar variants can occur in all parts of a left or right iliac vein. Stenosis exceeding 50%, especially with surrounding fibrotic scarring and significant features of CVD, may benefit from intervention.12 Up to 24% of the general population may demonstrate this potentially symptomatic variant with fibrotic scarring, yet only a small number develop this condition.10
Other causes
Benign and malignant lesions from an adjacent lymphadenopathy, uterine fibroids and cysts, or abdominal and pelvic cancers can lead to CVOO. Associated radiotherapy, central venous cannulation, trauma, and surgical treatment also may be implicated. Retroperitoneal fibrosis is treated pharmacologically, but endovenous intervention has been described for persistent venous symptoms.13 Congenital absences of deep veins such as inferior vena cava atresia and those associated with Klippel-Trenaunay syndromes are rare.14
THE INITIAL ASSESSMENT
The initial assessment for patients with CVOO is the same as for CVD. The patient’s symptoms and signs, associated with prolonged standing, worsen as the day progresses. Edema, skin changes, and ulceration tend to occur at the ankle where the venous pressure is at its highest in the blood column.
Even though no manifestations clearly point to CVOO as the cause of the patient’s CVD, several clinical features listed in Table 2 may increase clinical suspicion. Delis and colleagues reported15 that 43.6% of patients with prior iliofemoral DVT developed venous claudication during follow-up. Differential diagnoses include ankle-swelling secondary to cardiac, hepatic, or renal failure; skin infection; arterial, neuropathic, and diabetic ulcers; pelvic venous reflux; lymphedema; and malignancy. Detailed assessment of thrombotic risk factors for patients with a history of venous thromboembolism is essential.
Clinical features of chronic venous outflow obstruction
IMAGING: STRENGTHS AND LIMITATIONS
Duplex ultrasonography
Duplex ultrasonography is the first-line investigation for CVD of the lower limb, used to detect incompetence and obstruction of superficial and deep veins. It is noninvasive and economical and uses no ionizing radiation.
When CVOO is suspected, imaging of all the deep veins, including the iliac veins and inferior vena cava, is important. Imaging should demonstrate the presence of obstruction or significant reflux, or both. The presence of phasic flow in the common femoral vein may indicate that there is no significant CVOO.16 Phasic flow refers to the normal pulsation of the venous flow, reflecting the cardiorespiratory cycle. Significant CVOO can interrupt the continuity of the blood column. Transvaginal duplex ultrasonography can help diagnose or rule out pelvic venous reflux.
Despite its first-line role, duplex ultrasonography has relatively low sensitivity (67%) and specificity (70%).17 Among its limitations, duplex ultrasonography may provide an inadequate view of the iliac veins in approximately 20% of cases,.18 Views may also be inadequate in patients who have obesity or bowel gas,18 and operator skills and interoperator variability may affect the results.
Magnetic resonance and computed tomographic venography
Magnetic resonance venography and computed tomographic venography help to define the anatomy of the abdominal and pelvic veins and surrounding structures and assess for venous obstruction and dilation, and the presence of collateral veins. In CVOO, these imaging options help confirm the diagnosis and plan treatment,18 but neither technique is ideal. Nephrotoxic contrast is used in computed tomographic venography and contrast-enhanced magnetic resonance venography. Magnetic resonance venography protocols such as time-of-flight techniques and balanced steady-state free precession19 do not use contrast.
Computed tomographic venography also exposes patients to ionizing radiation. In a retrospective study, researchers found NIVL on conventional venography in 30.6% of patients with unexplained lower limb swelling and pain who had undergone nondiagnostic duplex ultrasonography, magnetic resonance venography, and computed tomographic venography.16 Magnetic resonance venography and computed tomographic venography are highly sensitive and specific for the diagnosis of iliocaval and iliofemoral DVT, but sensitivity appears to diminish in identifying CVOO.20,21
Ascending contrast venography
Ascending contrast venography, historically the mainstay technique for the diagnosis of CVOO, has been superseded by noninvasive duplex ultrasonography and computed tomographic and magnetic resonance venography. Contrast venography is now usually used in interventional procedures. The sensitivity of single-plane venography in detecting venous stenosis greater than 70% is reportedly only 45% despite the use of multiple views.22 Besides being invasive, ascending contrast venography is also limited by the use of nephrotoxic contrast and radiation.
Intravascular ultrasonography
Intravascular ultrasonography is regarded by many as the gold standard for the detection of CVOO. The technique, which uses an ultrasound probe at the tip of a catheter, delineates intravenous lesions better than other venographic techniques,22 especially if there are intraluminal webs that would not otherwise be visible. In the Venogram vs IVUS for Diagnosing Iliac vein Obstruction (VIDIO) trial, intravascular ultrasonography identified significant lesions not detected by 3-view venography in 26.3% of patients.23 The findings led to a revision of treatment plans in 72% of cases.23 Further, clinical improvement after stenting was best predicted by the stenotic area measured at baseline by intravascular ultrasonography, with 54% estimated as the optimal stenosis threshold for interventional treatment.24
Other important roles of intravascular ultrasonography include treatment planning, sizing and placement of stents, and detection of in-stent restenosis.21,22 However, it is invasive and costly.
MANAGEMENT STRATEGIES
The objective of treating CVOO is to reduce the risk of PTS and can range from compression therapy to surgical revascularization. Whatever treatment strategy is indicated, close follow-up is part of the management plan.
First steps
Early and adequate administration of therapeutic anticoagulation and adherence to therapy after an episode of acute DVT are associated with a decreased incidence of PTS.25 Other preventive measures, although not proven, include wearing compression hosiery26 and walking and exercising as soon and as much as the patient is able.25 These measures reduce the propagation of thrombus and recurrence of DVT, and they improve recanalization of the obstructed veins, reducing the risk and severity of PTS.25,26
Early thrombolysis
Early removal of thrombus in DVT re-establishes patency and reduces inflammatory processes caused by the heavy thrombus load that can lead to valvular damage and vein-wall fibrosis. Theoretically, this reduces the risk of PTS.
For iliofemoral DVT, there is conflicting evidence to support catheter-directed or pharmacomechanical thrombolysis in appropriate patients. These strategies are associated with a reduced risk of developing severe PTS but an increased risk of bleeding.27,28 Systemic thrombolysis is rarely used. Widely recognized guidelines, including those from the National Institute for Health and Care Excellence (NICE),29 the European Society for Vascular Surgery,30 the Society for Vascular Surgery, and the American Venous Forum,31 recommend consideration of early endovascular removal of thrombus for selected patients with iliofemoral DVT. The patient criteria for thrombolysis of acute iliofemoral DVT recommended by NICE, and similar to other organizations’ guidelines, are:
Symptoms lasting less than 14 days
Good functional status
A life expectancy of 1 year or more
A low risk of bleeding.
After clearance of thrombus, diagnostic venography and intravascular ultrasonography can be performed to assess for an underlying lesion. If an underlying lesion is found, balloon angioplasty with potential stenting can decrease the risk of reocclusion and the development of CVOO.
Conservative measures
A large, randomized control trial demonstrated no superiority of compression therapy over no compression therapy.32 Nevertheless, graduated compression therapy remains standard practice for the treatment for CVD and CVOO. Graduated compression stockings improve venous return and microcirculation by increasing the efficiency of venous flow and emptying of the lower limb through external pressure.33 Multilayered compression bandaging may be required to aid ulcer healing. Patients should be counseled to remain mobile, exercise, elevate their legs at rest, and lose weight. Prolonged standing increases columnar venous pressure and should be avoided. Some patients may need to consider significant lifestyle changes, including occupational adjustments or even a change of jobs.
Next step: Endovenous intervention
If conservative measures do not relieve the patient’s symptoms, then endovenous intervention (Figure 3) should be considered before open surgical revascularization. Shared decision-making with the patient includes discussion of the benefits and risks of intervention compared with no intervention, the need for long-term surveillance, potential secondary interventions, and the importance of adherence—possibly long-term—to a period of anti coagulation therapy. Endovenous stenting has been used in a significant number of cases only in the last 5 to 10 years, so long-term surveillance and outcome data are lacking. Nevertheless, stenting is an essential step, as balloon angioplasty alone disrupts the fibrotic tissues of the obstruction but is insufficient to maintain luminal patency.34
Contrast venography and intravascular ultrasonography of a 44-year-old man with obstructed left iliofemoral vein secondary to postthrombotic syndrome just before and after stenting. (A) Prestenting contrast venography shows complete obstruction of the left iliofemoral vein. The venous return of the left leg is through collateral veins (black arrow). (B) Poststenting contrast venography shows patent left iliofemoral vein following balloon angioplasty and stent placement with disappearance of the collateral veins. (C) Prestenting intravascular ultrasonography of the left common iliac vein shows that the vein (white arrow) is obstructed and compressed by the right common iliac artery (RCIA). (D) Poststenting intravascular ultrasonography of the left common iliac vein (LCIV) at the same level as in C shows the lumen of the vein is patent and maintained by the stent (white arrow). (IVC = inferior vena cava; LCFV = left common femoral vein; LEIV = left external iliac vein)
Growing evidence from nonrandomized clinical trials, including controlled prospective interventional studies and registries, supports the clinical efficacy and safety of endovenous intervention for CVOO. A double-blind randomized clinical trial compared medical treatment vs iliac vein stenting in 207 CVD patients with a median follow-up of about a year.35 Endovascular treatment was safe and beneficial for symptom relief and quality of life.35 For example, recanalization of the CVOO with stents achieved significant improvement in pain and swelling, venous ulcer healing rate, disease severity scores (such as the Venous Clinical Severity Score and Venous Disability Score), and health-related quality-of-life measures.
A recent meta-analysis of 16 single-arm observational studies of endovenous stenting included 1,688 patients, 70.5% with PTS and the rest with NIVLs.36 The reported primary patency ranged from 59% to 94%, and secondary patency ranged from 87% to 100%.36 Encouraging data are also emerging for the long-term patency rate of endovenous stenting of CVOO.37 Further, major societies and organizations support its use. The Cardiovascular and Interventional Radiological Society of Europe, the Society for Vascular Surgery, and the American Venous Forum recommend endovenous stenting for severe CVOO.38,39 The American Heart Association40 assigned a class IIb recommendation with evidence level B to endovenous stenting for CVOO, while the European Society for Vascular Surgery recommendation is class IIa with evidence level C.41
When to consider surgery
Open surgical bypass and reconstruction of deep veins are invasive procedures with significant morbidity risks, highly varied patency rates, and limited evidence.42 Open surgical revascularization of CVOO should be considered only as a last resort in highly selected patients whose CVD symptoms remain severe despite conservative measures and endovascular intervention.
Follow-up and antithrombotic strategies
Poststenting surveillance is vital to ensure that significant in-stent restenosis and thrombosis are detected and treated early, while optimal antithrombotic therapy is continued to prevent or reduce these risks. Poststenting surveillance and antithrombosis are often based on society guidelines, consensus statements, local multidisciplinary teams, and the individual clinician’s preference and experience. Seshadri Raju, MD,43 a pioneer in iliofemoral stenting, suggests surveillance with duplex ultrasonography the day after the procedure, and again at 4 weeks, 3 months, and yearly thereafter. A recent multidisciplinary consensus acknowledged highly varied practices across institutions, but recommended intensive follow-up duplex ultrasonography in the first 6 months after endovenous stent placement: ie, at 2 weeks, 6 weeks, 3 months, 6 months, and annually thereafter, especially in the case of thrombotic lesions.12
NIVLs may require less-intense surveillance if early in-stent complications are not present. Most clinicians consider reintervention if in-stent restenosis occurs in more than 50% of the luminal area or if CVD symptoms deteriorate.
Like many other clinicians, we use therapeutic-dose low-molecular-weight heparin for the first 2 to 6 weeks after stenting. We then convert to a direct oral anticoagulant if surveillance duplex ultrasonography shows no significant in-stent restenosis and the patient’s symptoms improve. Some clinicians may use antiplatelets alone for NIVLs. Longer-term antithrombotic strategies—varying in type, intensity, and duration—often depend on the patient’s risk of venous thromboembolism. Overall, the intensity and duration of poststenting antithrombotic therapy is decreased for NIVL over PTS. In complex PTS cases, a multidisciplinary approach, including a hematology consult, is essential.
REFERRAL AND INTERVENTION
Patients seek medical attention for CVD through varying routes and with various caregivers. They may consult first with primary care physicians and nurse practitioners who refer them to vascular specialists, dermatologists, and plastic surgeons. Some patients who develop PTS are already being followed for DVT by a vascular or hematologic clinician. Many clinics that specialize in leg ulcers are managed by nurses or allied healthcare professionals. For many patients with PTS, the index event was likely unrecognized by the patient or clinician, or was treated and the patient was then lost to follow-up. We are all aware of patients who present for the first time with CVD-associated skin changes and ulceration. In some instances, superficial venous incompetence is assessed and treated ahead of or simultaneously with CVOO management.
Although no clear evidence supports strict criteria for pursuing advanced imaging and referral for consideration of intervention,10 it is generally recommended that patient selection for intervention consider severity of symptoms, failure of conservative measures, superficial venous reflux therapy, and episodes of recurrence, as well as age and general frailty. While there is no evidence that duration of the ulcer or severity of symptoms determines likelihood of successful intervention to relieve CVOO, we believe that patients with the most severe symptoms are likely to achieve the most clinical benefit.
TAKE-HOME MESSAGES
CVOO, especially secondary to NIVLs and PTS, is increasingly recognized as an important cause of CVD. Growing evidence shows that endovascular intervention for CVOO is effective and safe. It achieves acceptable patency rates in many patients with severe CVD when conservative measures and treatment of superficial venous incompetence alone fail to relieve symptoms.
Patients with CVD—particularly those whose symptoms of CVD are inadequately relieved by conservative measures and treatment of superficial venous incompetence resistant to initial intervention—should be referred to a vascular center with experience in deep venous intervention for assessment and management of CVOO.
DISCLOSURES
Dr. Chung Sim Lim has disclosed receiving speaker fees from Boston Scientific. Dr. Harris reports no relevant financial relationships which, in the context of his contributions, could be perceived as a potential conflict of interest.
- Copyright © 2021 The Cleveland Clinic Foundation. All Rights Reserved.