A 60-year-old man with stage IV prostate cancer arrived at the emergency department 45 minutes after suddenly losing the ability to speak.
He had received his cancer diagnosis 12 years earlier. At that time, the tumor was still confined to the prostate, and he had undergone prostatectomy followed by adjuvant radiation and leuprolide treatment to block testosterone production. He did well for about 8 years, but then was found to have multiple bone metastases, consistent with stage IVB. His treatment was changed to leuprorelin, abiraterone (an agent that blocks the cytochrome P450 enzyme CYP17 expressed in tumor cells, thereby inhibiting androgen biosynthesis), and prednisone.
His prostate-specific antigen level had been rapidly rising: it had been 60 ng/mL 2 months ago, rising to 200 ng/mL 2 weeks ago. His oncologist had planned to start the immunologic agent sipuleucel-T, which is thought to work through antigen-presenting cells to stimulate a T-cell immune response targeted against prostatic acid phosphatase, which is highly expressed in most prostate cancer cells.1 However, this treatment had not yet been started.
He had no history of heart valve disease, arrhythmias, coagulopathy, or bleeding diathesis and was not receiving anticoagulation or antiplatelet therapy.
INITIAL EXAMINATION AND STUDIES
The patient’s blood pressure was 198/101 mm Hg, pulse 94 beats per minute, respiratory rate 22 per minute, and temperature 98.6°F (37.0°C). He was alert but unable to follow commands.
On neurologic examination, he had right-sided neglect (ie, he did not respond to stimuli on the right side of his body) and global receptive and expressive aphasia (ie, he could not speak, and he did not seem to understand us when we spoke to him). He could move all 4 limbs spontaneously without limb drift. The deep tendon reflexes in the upper and lower limbs were 1+ on a scale of 0 (completely absent) to 4+ (clonus) and symmetric. Babinski reflexes were not present. His score on the 42-point National Institutes of Health Stroke Scale was 15, indicating he was having a moderate stroke.
His heart rhythm was regular without gallops, murmurs, or rubs. The rest of the examination was normal.
Laboratory testing showed anemia, thrombocytopenia, elevated prothrombin time, and elevated alkaline phosphatase and troponin levels (Table 1).
The patient’s laboratory findings
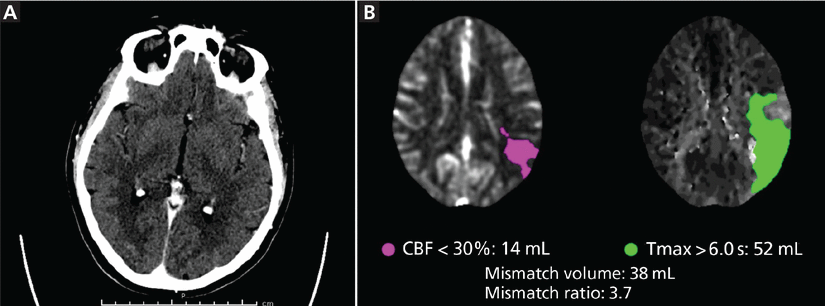
Computed tomography (CT) performed according to stroke protocol showed an acute thrombus in the M2 and M3 segments of the left middle cerebral artery and a subacute infarct in the right parieto-occipital area (Figure 1). The patient underwent thrombectomy, which restored perfusion completely (Thrombolysis in Cerebral Infarction [TICI] grade 3), and his aphasia and dysarthria resolved toward the end of hospital day 1.
(A) Initial noncontrast computed tomography (CT) shows no gross abnormalities. (B) The mismatch perfusion CT image shows abnormal perfusion in the left middle cerebral artery, inferior division distribution (CBF = cerebral blood flow; Tmax = time to maximum).
Transthoracic echocardiography indicated that his ejection fraction, wall motion, and heart valves were normal, and he had no intracardiac clots or shunts.
DAY 2: ANOTHER STROKE, ON THE OTHER SIDE
However, on hospital day 2, new signs appeared. The left side of his face was drooping, his left upper extremity was weak (his strength was graded 2 on a scale of 5 in the hand, 3 in the forearm, and 4 in the arm), with increased reflexes (2+) in the biceps, brachioradialis, and triceps, and he now had left-sided neglect with right-gaze preference—the opposite of the day before. His heart was still in sinus rhythm and remained so throughout his stay in the hospital.
Repeat CT now showed an acute thrombus in the right M1 segment, and he underwent a second thrombectomy, which restored perfusion (TICI grade 3). The retrieved thrombus had a gelatinous appearance, inconsistent with typical hematologic emboli. Magnetic resonance imaging, done after the procedure, showed new infarcts in the right frontal lobe and left occipital lobe.
The rapid succession of strokes involving different vascular territories suggested a thromboembolic phenomenon. Consultants in neurology, cardiology, and hematology-oncology together agreed it would have been pointless to start anticoagulation, in view of the patient’s poor prognosis due to prostate cancer.
CAUSES OF EMBOLIC STROKE
1. Of the following, which is the most common cause of cardioembolic stroke?
Bacterial endocarditis
Advanced heart failure
Atrial fibrillation
Right-to-left cardiac shunt
Stroke is classified as either hemorrhagic or ischemic, with ischemic stroke more common. Cardioembolic stroke is a subcategory of ischemic stroke.
Atrial fibrillation is common and is becoming more so. Estimates of its prevalence vary widely, but Colilla et al2 project that it will affect 12.1 million people in the United States by 2030. Owing to its high prevalence, it is the most common cause of cardioembolic stroke and may account for 15% of all strokes in the United States.3
In fact, atrial fibrillation may be causing even more strokes than we think. Recent studies suggest that undiagnosed paroxysmal atrial fibrillation accounts for a significant proportion of the 30% of strokes that are classified as embolic stroke of undetermined source.4 In this situation, patients may be experiencing episodes of atrial fibrillation, but not in the clinic or hospital. Implantable cardiac devices such as pacemakers and loop recorders have made it easier to diagnose paroxysmal atrial fibrillation and have helped establish that episodes of atrial fibrillation lasting at least 6 minutes increase the risk of stroke 2.5-fold for the subsequent 2.5 years.5
Advanced heart failure and recent myocardial infarction increase the risk of stroke 3-fold.6 The mechanism seems to be regional stasis due to wall-motion abnormalities associated with these 2 conditions and the hypercoagulable condition resulting from the inflammatory process triggered by transmural infarcts, leading to clots forming in the left ventricle.4 In addition, many patients with either of these conditions undergo percutaneous coronary angiography for diagnosis and treatment, which can rupture an aortic arch atheroma.4 Cardiac sources should be considered particularly in those under age 45 even without clinical evidence of advanced heart failure.
Bacterial endocarditis increases the risk of embolic stroke dramatically. Merkler et al7 reported that 2,275 (13%) of 17,926 patients with infective endocarditis had strokes in the year surrounding the diagnosis, with more than an 80-fold increase in risk in the first month compared with baseline.
Right-to-left shunt can allow venous thromboemboli to paradoxically enter the atrial arterial circulation and cause strokes. Although about one-fourth of adults have a patent foramen ovale, it does not appear to be a strong risk factor for stroke, except possibly in patients under age 50.4
Other causes of cardioembolic stroke include mechanical prosthetic heart valves, dilated cardiomyopathy, regional left ventricular akinesis, atrial myxoma, and rheumatic heart disease. Noncardiac causes of embolic stroke include aortic arch atheroma, carotid plaque, and, most relevant to our patient, malignancy.
Cancer as a cause of stroke
Cancer is a major cause of embolic stroke.8–10 About 10% to 15% of all patients admitted to a stroke service also had cancer, and for some, stroke was the initial symptom of cancer.8–10 In one study, the odds ratio of having undiagnosed cancer when an arterial thromboembolic event occurs was 1.69 (95% confidence interval 1.63–1.76).10 Although all types of stroke are seen in patients with cancer, embolic stroke of undetermined source accounts for about half of all ischemic strokes.8
The association of cancer with thromboembolic events, both arterial and venous, is not a new finding. Professor Armand Trousseau made the first observations of this phenomenon in the 1860s.11
The risk of stroke is particularly high in the year before cancer is diagnosed, the first 6 months after the diagnosis, and when cancers metastasize to distant sites.8 Neoplasias frequently associated with stroke include leukemias, lymphomas, and cancers of the lung, breast, pancreas, colon, rectum, kidneys, and prostate.8,9
Multiple mechanisms explain the association between cancer and stroke.8,9
Cancer cells invading the vascular system can trigger the coagulation cascade, activate platelets, or both.9
Some tumors are highly active in terms of protein production. Mucin in particular, which prostate cancer frequently produces, can mimic coagulation factors or make the plasma more viscous, triggering the coagulation cascade.9
Tumors can also mechanically compress large vessels, leading to blood stasis and clotting.9
Neutrophil activity and platelet activity are both increased in cancer, leading to platelet aggregation and coagulation cascade activation.8
Certain chemotherapies such as methotrexate, asparaginase, and cisplatin and cancer-supportive therapies such as colony-stimulating factors also increase the risk of stroke.9 Radiation may accelerate the process of atherosclerosis.8
Systemic thrombotic microangiopathy, which was first observed in autopsy studies, is another mechanism.9
DAY 3: BLEEDING
Hospital day 3 saw new trouble for our patient: mild bleeding from the nose and frank bleeding in the urine, the latter requiring placement of a 3-way Foley catheter with continuous bladder irrigation. He had not received any anticoagulation or antiplatelet therapy.
And another new sign was a holosystolic murmur (graded 2 on a scale of 6), loudest over the apex and increasing with expiration, indicating new mitral regurgitation. Cardiac telemetry still showed sinus rhythm.
Table 1 shows pertinent laboratory results obtained on that day. No schistocytes were seen on preliminary review of a peripheral smear specimen obtained on day 2 or on the final report of the smear, which was received on day 9.
DISSEMINATED INTRAVASCULAR COAGULOPATHY
2. Which of the following is the most common laboratory abnormality in disseminated intravascular coagulopathy (DIC)?
Thrombocytopenia
Low fibrinogen level
Prolonged prothrombin time
Elevated D-dimers
DIC is systemic activation of the coagulation system.9,12,13 Whether the insult that triggers it is an inflammatory process due to cancer, trauma, infection, or an autoimmune condition, the result is an imbalance between thrombus formation and thrombolysis that ultimately leads to consumption and exhaustion of these factors.12–14 Up to 15% of patients with cancer or major trauma and up to 40% of patients with sepsis (due to gram-negative rods in particular) present with DIC.13,14
DIC associated with malignancies is thought to be caused by tumor cells expressing procoagulant factors such as cysteine protease, which has factor X-activating properties.13 Also, mucin production, which is increased in prostate cancer, appears to play a role by increasing plasma viscosity.9 Almost all patients with advanced malignancies experience a procoagulant state that places them at risk for DIC.13
One of the major challenges in clinical practice is that DIC is frequently a subclinical condition, and no single symptom, finding, or test value confirms the diagnosis.12,13 Most patients who present with symptoms have widespread clotting resulting in various degrees of organ damage.13
Highly vascularized organs such as the liver, kidneys, spleen, lungs, and brain are more susceptible to occlusion of the microvasculature caused by the fibrin deposits.12–14 However, as coagulation factors and platelets are used up, a minority of patients experience bleeding as the predominant manifestation.13,14
Varied laboratory findings in DIC
No single test is diagnostic of DIC because, although each of them is highly sensitive, they lack specificity.12
Thrombocytopenia or a rapidly falling platelet count is seen in 98% of patients with DIC.12 Thus, it is the correct answer choice above. Extremely low platelet counts increase the risk of bleeding between 4-fold and 5-fold.12 However, half of patients have platelet counts higher than 50 × 109/L—ie, low, but not extremely low.12
Prothrombin times and activated partial thromboplastin times are prolonged in about half of patients.
Fibrinogen levels remain normal or even elevated in half of patients with DIC, because it is an acute-phase reactant.12
The International Society of Thrombosis and Hemostasis scoring system incorporates prothrombin time, platelet count, and D-dimer and fibrinogen levels (Table 2).15 It has a sensitivity and specificity of 95%, and high scores strongly correlate with risk of death.12,15,16
International Society of Thrombosis and Hemostasis scoring system for disseminated intravascular coagulopathy
DIC management
In general, DIC must be managed by correcting the underlying inflammatory process. In our patient, who had stage IVB prostate cancer, the underlying inflammatory state was irreversible. Treatment for DIC associated with malignancy includes supportive treatment with platelet transfusion (aiming at a platelet count higher than 30 to 50 × 109/L), fresh frozen plasma, and fibrinogen concentrate (guided by the fibrinogen concentration in the patient’s plasma). The use of heparin does not have enough data to support it.14
A NEW MITRAL VEGETATION, MULTIPLE INFARCTS
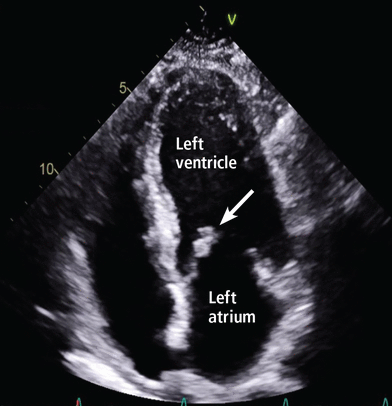
In our patient, repeat echocardiography showed a new mobile mass measuring 0.6 by 0.8 cm on the anterior mitral leaflet, causing moderate regurgitation (Figure 2).
Transthoracic echocardiography showed a new mitral valve vegetation (arrow) on the apical four-chamber view.
The patient had no physical findings to suggest bacterial endocarditis. Furthermore, 2 sets of blood cultures were obtained, and they remained negative. The opinion of cardiology and infectious disease physicians was that the patient had nonbacterial thrombotic endocarditis.
CT of the chest, abdomen, and pelvis revealed infarcts in the kidneys and spleen and multifocal osseous metastases in the pelvis and several vertebrae. The cardiology and hematology-oncology consultants agreed that the multiple infarcts involving the kidneys and spleen were consistent with DIC.
Because the patient’s hemoglobin level was low and falling, he was given a blood transfusion, and his fibrinogen level was monitored with the intention of giving him cryoprecipitate if the level dropped below 100 mg/dL. Unfortunately, his hematologic values did not improve (Table 2),15 and he became increasingly tachypneic, with persistent epistaxis requiring intubation to protect his airway.
He had intermittent episodes of supraventricular tachycardia and suffered an anterior myocardial infarction with pulmonary edema (Killip class III). Further, new neurologic signs arose, prompting repeat CT of the head, which showed hemorrhagic transformation of the subacute strokes.
The patient did not have an advance directive in place before his admission. However, he did sign a medical power of attorney form during the hospital stay naming a family member to make surrogate decisions for him if he lacked capacity to make them. The decision was made with this family member and the rest of the family to move to comfort care. The patient died shortly thereafter.
NONBACTERIAL THROMBOTIC ENDOCARDITIS
3. What is the most common cause of noninfectious endocarditis?
Systemic lupus erythematosus
Congenital valve abnormalities
Malignancy
Blood culture-negative endocarditis
Malignancy is the cause of 78% to 80% of cases of nonbacterial thrombotic endocarditis, mostly cancers of the pancreas, lungs, or stomach and adenocarcinomas of unknown origin.17,18 However, the literature is limited to 2 autopsy series.17,18 Deppisch and Fayemi,17 in a 1976 autopsy study of 65 patients with nonbacterial thrombotic endocarditis, reported that adenocarcinoma was the most common histologic type of cancer associated with this condition, and 18.5% had findings suggestive of DIC. As mentioned previously, prostate cancer produces proteins such as mucin that create a hypercoagulable state.13
The mitral valve is affected in about two-thirds of patients, while the aortic valve is involved in one-fourth, and both valves are compromised in a minority of cases.17,18 Compared with the vegetations in bacterial endocarditis, those of nonbacterial thrombotic endocarditis are more friable and more likely to become dislodged.19 Thus, patients with nonbacterial thrombotic endocarditis are more likely to experience systemic embolization to the brain, spleen, and kidneys. For instance, embolic strokes occurred in 27% of patients (8 of 30) with nonbacterial thrombotic endocarditis in 1 series,20 compared with 21% (25 of 133) with bacterial endocarditis in another series.21
In our patient, the acute presentation of mitral regurgitation was consistent with nonbacterial thrombotic endocarditis.
Blood culture-negative endocarditis is not the same as nonbacterial thrombotic endocarditis. Blood cultures can remain negative in 2% to 40% of all cases of endocarditis.22 Negative blood cultures can result from giving antibiotics before blood samples for cultures are obtained or from infection with fastidious organisms such as Bartonella and Mycoplasma.22 Molecular techniques such as polymerase chain reaction are increasingly being used in diagnosing blood culture-negative endocarditis.22
Systemic lupus erythematosus and antiphospholipid syndrome are other causes of nonbacterial thrombotic endocarditis.19,23,24 About 11% of patients with lupus have evidence of nonbacterial thrombotic endocarditis or Libman-Sacks endocarditis, a form of nonbacterial thrombotic endocarditis seen in lupus. The mechanisms causing the valve damage, which eventually lead to formation of a vegetation, include deposition of immunoglobulins and complement factors in the case of lupus and formation of antibodies against the phospholipids of the endothelium in the case of antiphospholipid syndrome.19 In patients with lupus, the risk of nonbacterial thrombotic endocarditis is correlated with the duration of the lupus and is associated with the presence of antiphospholipid syndrome, although the latter is not necessary.19
Congenital valvular abnormalities such as a bicuspid aortic valve are a risk factor for bacterial endocarditis but not for nonbacterial thrombotic endocarditis.25 The endothelial damage caused by the congenital defect and subsequent turbulence facilitates adhesion of bacteria whenever an organism reaches the bloodstream.25
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.